By using advanced microscopes and imaging technology to study tiny pockets of liquid trapped in ancient minerals, scientists have gained new insights into how seawaters have changed over time, and how they might do so in the future. The research centers on small samples of seawater sealed away for 390 million years, and could inform not just the field of climate science, but open up new possibilities in the safe storage of hydrogen as a clean source of energy.
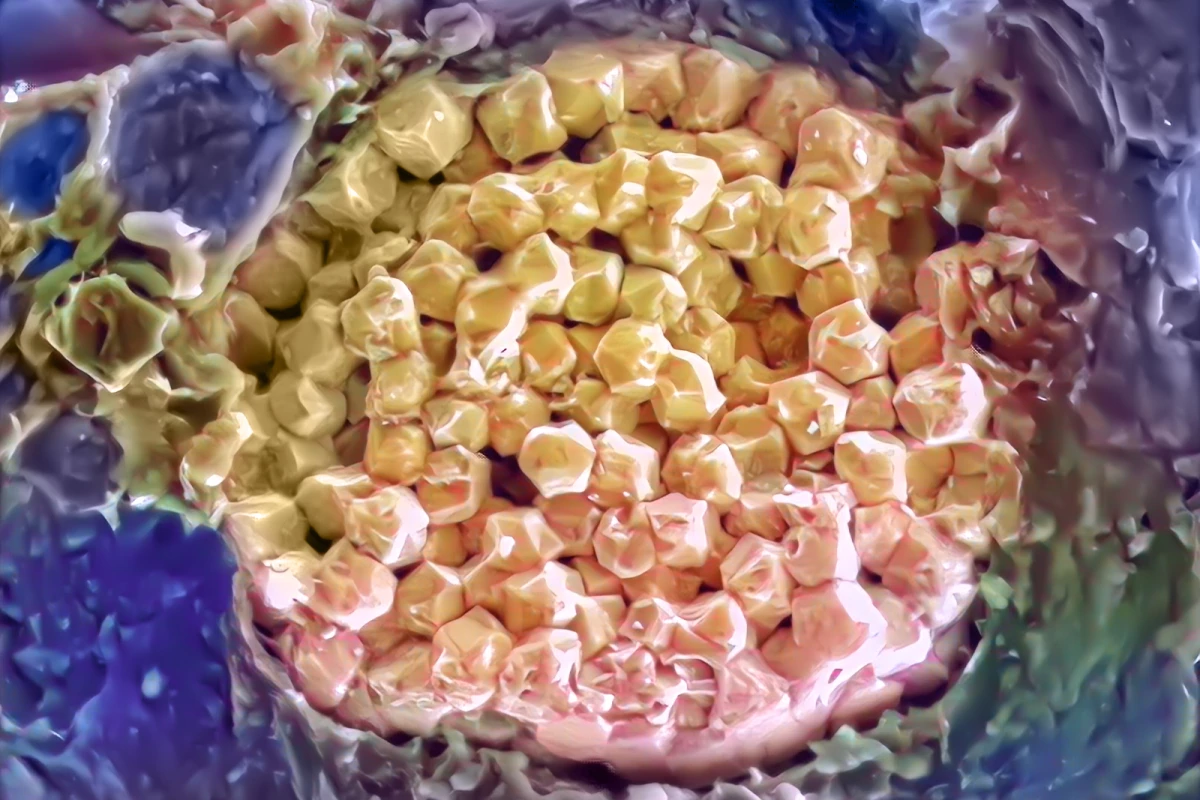
The breakthrough actually came about by chance, with a research team from the US and Canada originally studying the way arsenic leaches from a mineral called pyrite. These are also described as framboids, derived from the French word for raspberry, due to their resemblance to the fruit under the microscope. This close inspection led the team to identify tiny defects in their samples, which turned out to be small bubbles like those you might see trapped in a gemstone.
“We looked at these samples through the electron microscope first, and we saw these kind of mini bubbles or mini features within the framboid and wondered what they were,” Sandra Taylor, first author of the study and a scientist at the Department of Energy’s Pacific Northwest National Laboratory.
The team then turned to a combination of tools in order to find some answers, including mass spectrometry and atom probe tomography, enabling them to study the nanoscale features of the bubble and put together a chemical profile of the liquid. This marks a step forward for this type of chemical analysis, with the team able to confirm that the bubble contained seawater and its salt had a certain set of characteristics.
“We discovered we can actually dig out information from these mineral features that could help inform geologic studies, such as the seawater chemistry from ancient times,” said Taylor.
This analysis confirmed that the water locked away within the pyrite for 390 million years matched the chemistry of an ancient inland sea in the region from which it was sourced. This body of water in upstate New York stretched from current-day Michigan to Ontario in Canada, and featured vast coral reefs and sea scorpions the size of pickup trucks.
The findings therefore validate the team’s technique for accurately characterizing the contents of these types of ancient water bubbles, which could help fill in large blanks in the geological record. Mineral deposits can help scientists calculate historical temperatures or other features of the ocean but some of them come easier than others. The relative abundance of pyrite gives the scientists optimism when it comes to filling in important details.
“We use mineral deposits to estimate the temperature of the ancient oceans,” said Daniel Gregory, a geologist at the University of Toronto, and one of the study leaders. “Salt deposits from trapped seawater (halite) are relatively rare in the rock record, so there are millions of years missing in the records and what we currently know is based on a few localities where there is halite found. Sampling with this technique could open up millions of years of the geologic record and lead to new understanding of changing climate.”
In addition to furthering the field of climate science, the scientists say the research also lays the groundwork for new technologies that can safely store hydrogen or other gases underground in geological reservoirs. The tiny, light molecules that make up hydrogen make it an incredibly difficult gas to store and are a real roadblock in its widespread adoption as an energy source, but a detailed understanding of its interplay with rocks could lead to new solutions to this problem.
“Hydrogen is being explored as a low-carbon fuel source for various energy applications," said said Taylor. "This requires being able to safely retrieve and store large-amounts of hydrogen in underground geologic reservoirs. So it’s important to understand how hydrogen interacts with rocks. Atom probe tomography is one of the few techniques where you can not only measure atoms of hydrogen, but you can actually see where it goes in the mineral. This study suggests that tiny defects in minerals might be potential traps for hydrogen. So by using this technique we could figure out what’s going on at the atomic level, which would then help in evaluating and optimizing strategies for hydrogen storage in the subsurface.”
The research was published in the journal Earth and Planetary Science Letters.