Electrolyte
-
While cutting back on salt intake has long been a mainstay in treating high blood pressure, new research suggests that upping potassium intake might have a greater effect. It might be time to stock up on bananas, apricots, and sweet potatoes.
-
Rubber might not seem like a great candidate for an electrolyte material in a battery, but Georgia Tech researchers have developed a new rubbery material with a high conductivity, which could make for safer electric vehicle batteries with longer range.
-
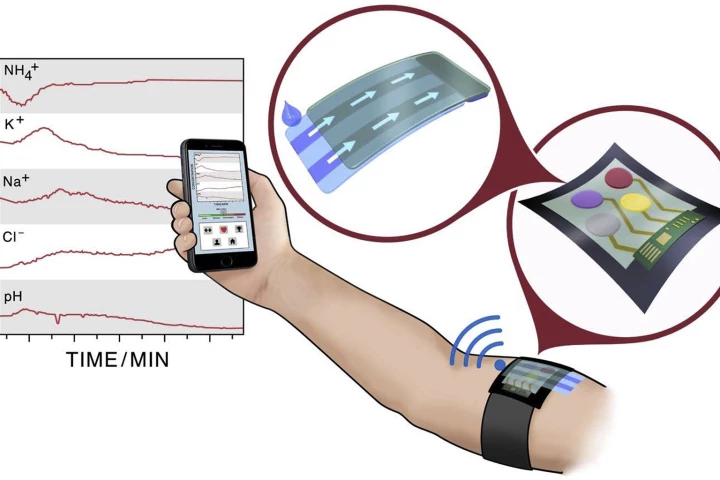
Wearable health-monitors are everywhere, from Fitbits for the health conscious to continuous glucose monitors for diabetics, but most are limited in what they can tell us, and there are issues around accuracy, calibration and reliability. Researchers in Sweden are working to change that.
-
San Francisco's Sufferfest Beer Company has come up with a new brew it claims boasts the electrolytes of some typical sports drinks, with a juicy superfood acting as the nutritional cherry on top.
-
We’re so used to having devices powered by lithium-ion batteries that it’s hard to imagine using anything else, but there are plenty of alternatives. The latest, an aqueous hybrid capacitor, is stable, safe and boasts high energy and power densities, recharging in as little as 20 seconds.
-
He’s the 94-year-old co-inventor of the lithium-ion battery. She’s a physicist with a less-than-conventional idea of building a better battery. Together, can they develop the technology that will finally deliver us from fossil fuels and kickstart the promised EV revolution?
-
While cheap, kerosene lamps are bad for the environment and human health. Intended as an alternative in areas with access to electricity, the SALt (Sustainable Alternative Lighting) lamp burns for eight hours at a time running on only a glass of water and two teaspoons of salt.
-
Dendrites – thin conductive filaments that form inside lithium batteries – reduce the life of these cells and are often responsible for them catching fire. Now researchers claim to have produced an electrolyte that completely eliminates them while also boosting carrying capacity and efficiency.
-
A new redox flow battery developed at the Pacific Northwest National Laboratory (PNNL) more than doubles the amount of energy that this type of cell can pack in a given volume, approaching the numbers of lithium-ion batteries.
-
Engineers at Penn State University have produced an ammonia-based battery that not only captures and converts waste heat into electricity economically and efficiently, but is also claimed to do so at a greater power capacity than other similar systems.
-
New research from the Karlsruhe Institute of Technology (KIT) details the development of an electrolyte that can be used in new magnesium-sulfur battery cells that would be more efficient and inexpensive than the dominate types of batteries today.
-
A team of researchers is seeking to challenge the theory that the anode, cathode and electrolyte of a battery can only work independently, experimenting with a dual functioning electrolyte that supplements the cathode, resulting in a significant improvement of the battery's capacity and lifespan.
Load More