Victims of penetrating head injuries usually seek immediate attention, as the hole in their skull is difficult to miss. However, people with closed-head injuries may show few immediate signs of the trauma, and appropriate diagnostic equipment (primarily a CAT scanner) is often not immediately available. A Mexican-US team of researchers has now developed a simple, easy to operate, and inexpensive electromagnetic sensor for traumatic brain injuries, suited to on site use by field personnel and paramedics.
Traumatic brain injury (TBI) is a common feature of life around the world, causing some 60,000 deaths each year in the US alone. The primary causes of TBI include shootings, falls, and traffic accidents. Of additional concern has been the recent discovery of the long-term effects of mild but repeated TBI, usually in the form of concussions, in participants of sports such as football, hockey, and martial arts. Overall, about 20 percent of TBI lead to death within a month, with many others resulting in permanent brain dysfunction.
Physicians believe many of these negative outcomes could be prevented by earlier diagnosis and treatment, ideally in the so-called "golden hour" immediately following the injury which offers the greatest chance of minimizing brain damage. Unfortunately, when injuries occur in the field, or are not clearly seen as serious conditions, evaluation by CAT scan is often delayed far beyond the golden hour.
Dr. Cesar Gonzales, of Mexico's Instituto Politécnico Nacional, Escuela Superior de Medicina, was particularly concerned about the lack of medical imaging in rural areas and economically disadvantaged areas of the world. He wanted to develop an inexpensive sensor with which medics in the field and ambulance paramedics could diagnose, with the help of remote physicians, situations that require some form of immediate treatment, and to provide notice of critical cases arriving at a hospital.
Noting that brain injuries usually produce characteristic changes in, for example, the amount of fluid in the brain, Dr. Gonzales, together with Professor of Bioengineering at UC Berkeley Boris Rubinsky, developed a method to measure the associated changes in the brain's electromagnetic properties. The result is a prototype Volumatic Electromagnetic Phase-shift Spectrometer (VEPS).

The VEPS sensor features two coils that are mounted on a helmet-like device to be worn by a patient. The smaller exciter coil emits radio waves into the top of the skull, and the larger coil is the sensor antenna. When radio waves are broadcast from the smaller coil, the signals received by the sensor antenna are modified by their interaction with the patient's brain. The system comes adjusted so that no signal is received from a normal brain.
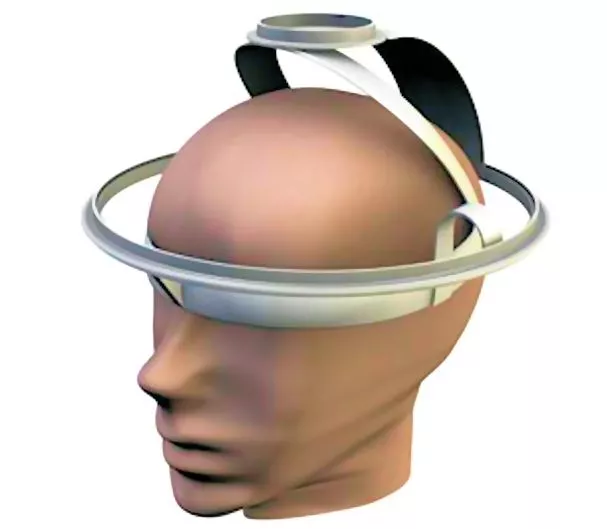
A commercial off-the-shelf (COTS) digital synthesizer is used to activate the exciter coil at 200 frequencies between 1 and 200 MHz. The signal received at each frequency is measured using COTS phase and amplitude sensitive electronics. Any signal which appears is then evaluated using computer analysis to decide what form and extent of brain trauma may have occurred. The test is rapid, and only requires the sensor and a hand-held electronics package. The list price for the sensor electronics is less than US$100, which will make the brain injury sensor widely available.
A small-scale clinical study of the VEPS sensor was undertaken with the assistance of the Critical Care Unit of the Mexican Army Military Hospital. VEPS readings from 46 healthy volunteers between the ages of 18 to 48 years were compared with those of eight patients aged 27 to 70 who were admitted for brain injuries resulting from infection, intracranial bleeding, or TBI. These patients were further assigned to one of two groups; those showing diffuse swelling as the main abnormality, and those showing local bleeding. CT images of the brain injuries of the eight patients appear in this article's photo gallery.
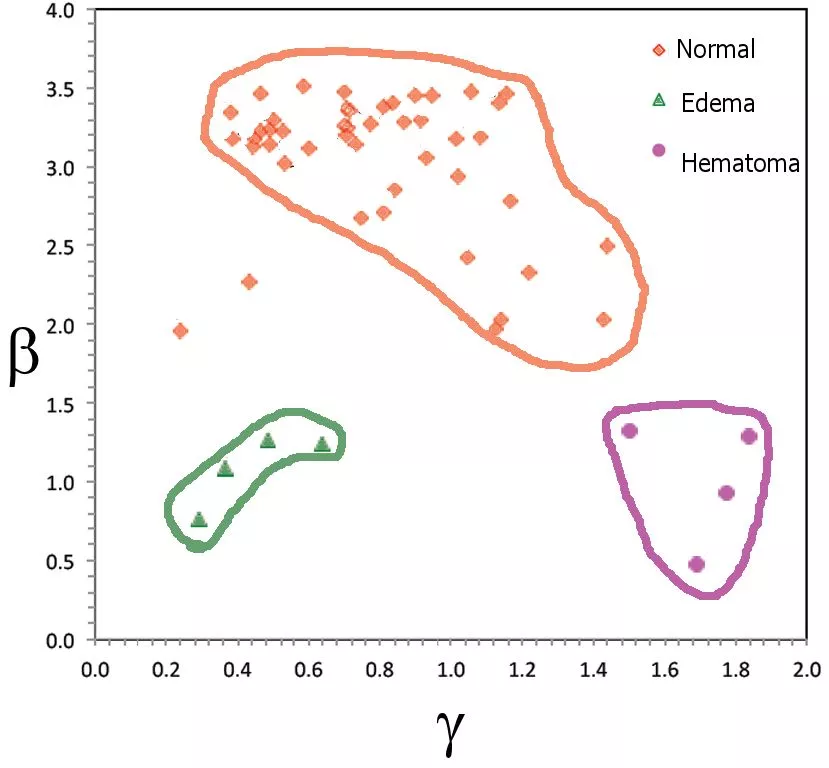
The result of a VEPS scan can be summed up in two parameters, β (beta) and γ (gamma), which are integrals of various sensor responses over applied RF frequency. As shown on the graph above, three groupings stand out clearly from the Mexican clinical study. Beta values greater than about 2.0 seem to indicate normal brain structure, more or less independent of the gamma value. (There are age-related correlations not shown here.)
In contrast, when beta is smaller than 2.0, some form of brain injury is indicated. The diffuse and localized injuries included in this study are clearly differentiated, with those TBI patients suffering diffuse edema producing gamma values considerably smaller than one, while for patients with local hematoma gamma was about 1.5 or larger.
While these early results are rather crude, the researchers say the device’s diagnoses for the brain trauma patients in the study matched the results obtained from conventional computerized tomography (CT) scans.
This suggests that the VEPS sensor can indeed act as a new instrument of field triage, to identify patients critically in need of immediate care so the medical rescue system can put them on a fast track toward treatment. Additionally, immediate diagnosis of sports-related head injuries would help protect our athletes in the harsher contact sports. It also seems possible that further development of VEPS as a diagnostic tool might result in a tool capable of finer distinctions, so that effective diagnosis of head injuries may not require access to a CT scanner.
In the absence of clear symptoms, brain injury is often missed until the damage is done. VEPS is likely a step toward prevention of permanent brain damage, rather than relying on rehabilitating such damage.
Sources: UC-Berkeley, PLOS ONE









