Filamon Limited, an Australian biotech company focused on developing next-gen anti-inflammatory drugs, has announced its breakthrough dementia treatment. ALPHA-003 is designed to preserve the integrity of vital brain cell structures and protect them against destruction caused by brain inflammation.
In healthy neurons, the tau protein stabilizes microtubules, crucial tube-like structures that, together with neurofilaments, maintain the neurons’ shape and provide mechanical support. However, when it’s modified, tau can form toxic aggregates – tangles that degrade these key structures. This is seen in a group of diseases called tauopathies, such as dementia, including Alzheimer’s disease, and chronic traumatic encephalopathy (CTE).
Existing dementia treatments focus on reducing the consequences of this structural damage but have enjoyed only moderate success. Now, an Australian biotech company, Filamon Limited, has announced its breakthrough treatment, ALPHA-003, which is aimed at halting the progression of dementia by preventing microtubular destruction.
“The underlying problem with most forms of dementia is the destruction of a key structural component of brain cells known as microtubules,” said Associate Professor Kieran Scott, Professor of Oncology at Western Sydney University, and co-founder of Filamon. “These long, hollow tubes are vital to healthy brain function. In dementia, these microtubules degrade, resulting in the death of brain cells.
“To date, no one has found a way of preventing microtubular destruction,” Scott said. “We believe ALPHA-003 has the potential to be that first drug by stabilizing the two main brain cell components whose job is to protect microtubules from damage – tau and neurofilaments.”
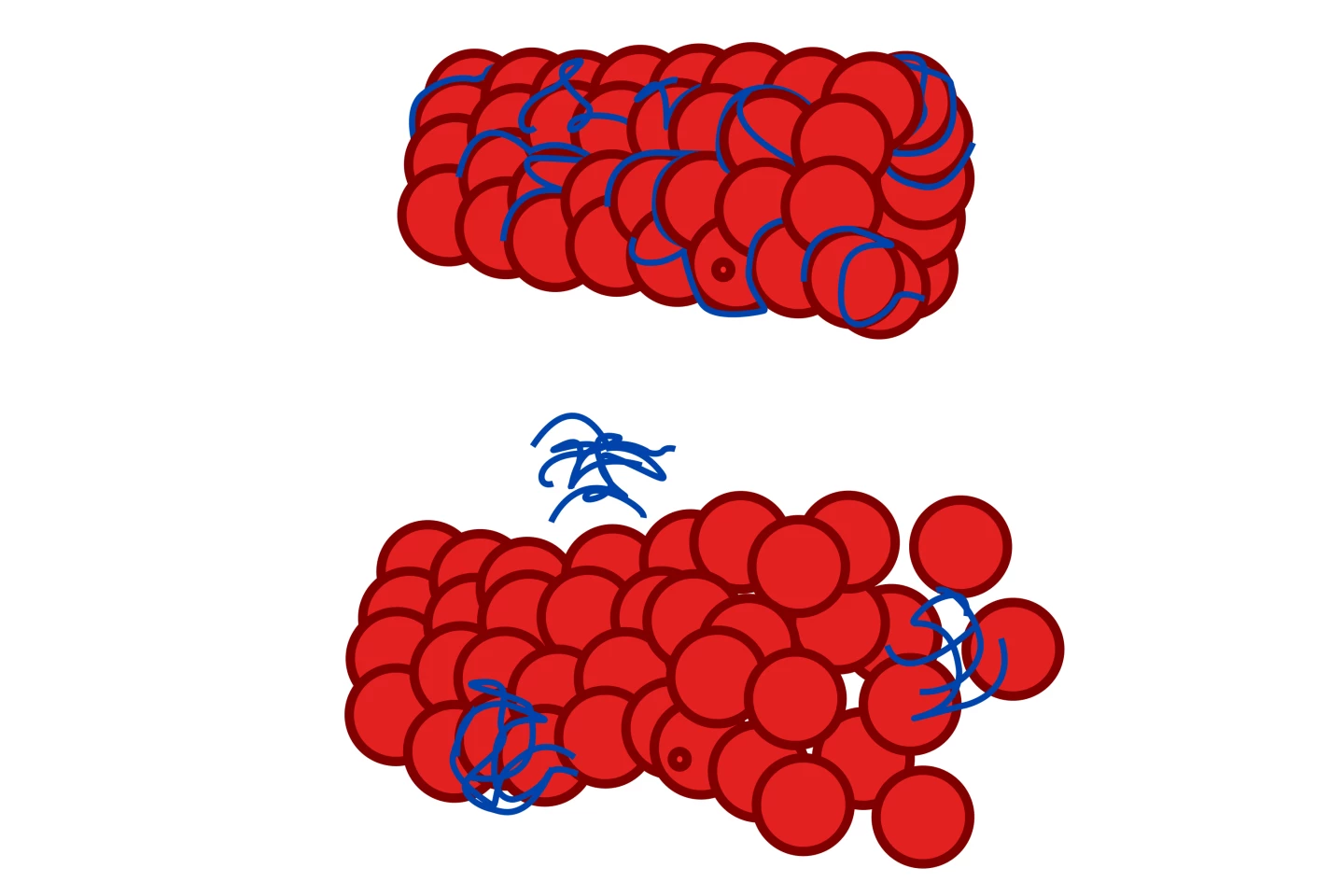
ALPHA-003 is designed to prevent damaging brain inflammation by binding to tau and neurofilaments, providing the microtubular protection that Scott is referring to. The result of deep-learning, computational drug design technology developed in Australia, ALPHA-003 started life as a more general anti-inflammatory drug, countering the effects of human group IIA secretory phospholipase A2 (hGIIA), a significant player in inflammatory conditions, before its developers realized its potential for treating neuroinflammation, specifically.
“ALPHA-003 was under development as a new form of anti-inflammatory drug that worked by blocking the activating effects of the key inflammatory ligand, hGIIA, on a range of cell structural proteins,” Professor Graham Kelly, Filamon’s co-founder, CEO, and Managing Director said in an interview with New Atlas. “Activation of those proteins underlies most chronic inflammatory changes. Recent published data has shown that tau is another structural protein that responds to hGIIA, so we simply asked the question whether ALPHA-003 would have the same protective effect on tau. Our studies showed that it does, to the extent of blocking the ability of tau to form sheets of oligomers that comprise the ‘tau neurofibrillary tangles.’”
But it wasn’t just the need to fill a treatment niche that drove the drug’s development. Kelly explained to New Atlas that experiencing the devastating effects of dementia first-hand was also a motivating factor.
“The personal angle has been the confronting experience of seeing a very good friend who has always been a bundle of energy and force of nature deteriorate into a tragic, sunken, shell of a woman who has lost all recognition of friends and family,” he said.
But does ALPHA-003 work? Pre-clinical studies strongly suggest that it does. Importantly, the drug has been found to cross the blood-brain barrier in mammals, meaning it can exert a direct effect on brain cells. The team at Filamon were so happy with the results that they announced them prior to journal publication.

“The announced news is literally freshly generated,” Kelly told New Atlas. “We considered it to be of such importance to warrant being released pre-publication. More studies are underway, and the results of those studies will be the subject of journal submissions.”
Kelly foresees ALPHA-003 being used to treat dementia at the time of diagnosis.
“We see dementia as a two-step process involving (i) creation of an ongoing neuroinflammatory trigger, and (ii) the consequences of that trigger,” he told New Atlas. “ALPHA-003 is aiming at blocking the latter, but that requires accepting that the underlying neuroinflammation trigger continues unabated. ALPHA-003 simply is aiming to mitigate the effects of the trigger. Another Filamon experimental drug program underway with another drug technology aims to deactivate this trigger.”
Filamon aims to have ALPHA-003 approved for clinical trial use in 2026. It’s expected that the drug will treat tauopathies other than the two major forms of dementia – Alzheimer’s disease and frontotemporal dementia – such as progressive supranuclear palsy, an uncommon Parkinson’s-like disorder, and CTE, caused by repeated concussions.
Source: Filamon [PDF]







