Using an existing steroid-free topical foam daily significantly improved the signs and symptoms of psoriasis on the scalp and the body, according to a recent clinical trial. It could be a game-changer for people with the condition in both places.
Treating psoriasis, a chronic inflammatory skin condition characterized by thick, scaly, red patches that can be itchy and painful, can be challenging if it appears on both the body and the scalp. Many topical treatments for the body aren’t suitable for use on the scalp because of the presence of hair, necessitating separate prescriptions.
However, a recently published Phase 3 clinical trial investigated the use of roflumilast foam, sold as Zoryve, as a single treatment for psoriasis on both the body and scalp, and produced some promising results.
Roflumilast reduces inflammation by targeting an enzyme called phosphodiesterase 4 (PDE4), which is involved in controlling the body’s inflammatory process. Importantly, it is steroid-free, which means it avoids side effects associated with steroids and is meant for long-term use. A foam version of the drug is indicated for the treatment of seborrheic dermatitis, a common skin condition causing scaly patches, inflamed skin and stubborn dandruff.
Like with seborrheic dermatitis, inflammation is a problem in psoriasis. The condition causes the immune system to go into overdrive, resulting in the characteristic plaques that result from the buildup of skin cells. In addition to dampening this immune response, roflumilast inhibits sensory nerve cells, reducing itching. The trial investigators wanted to see if the drug was an effective psoriasis treatment because of its immune-dampening and itch-reducing effects.
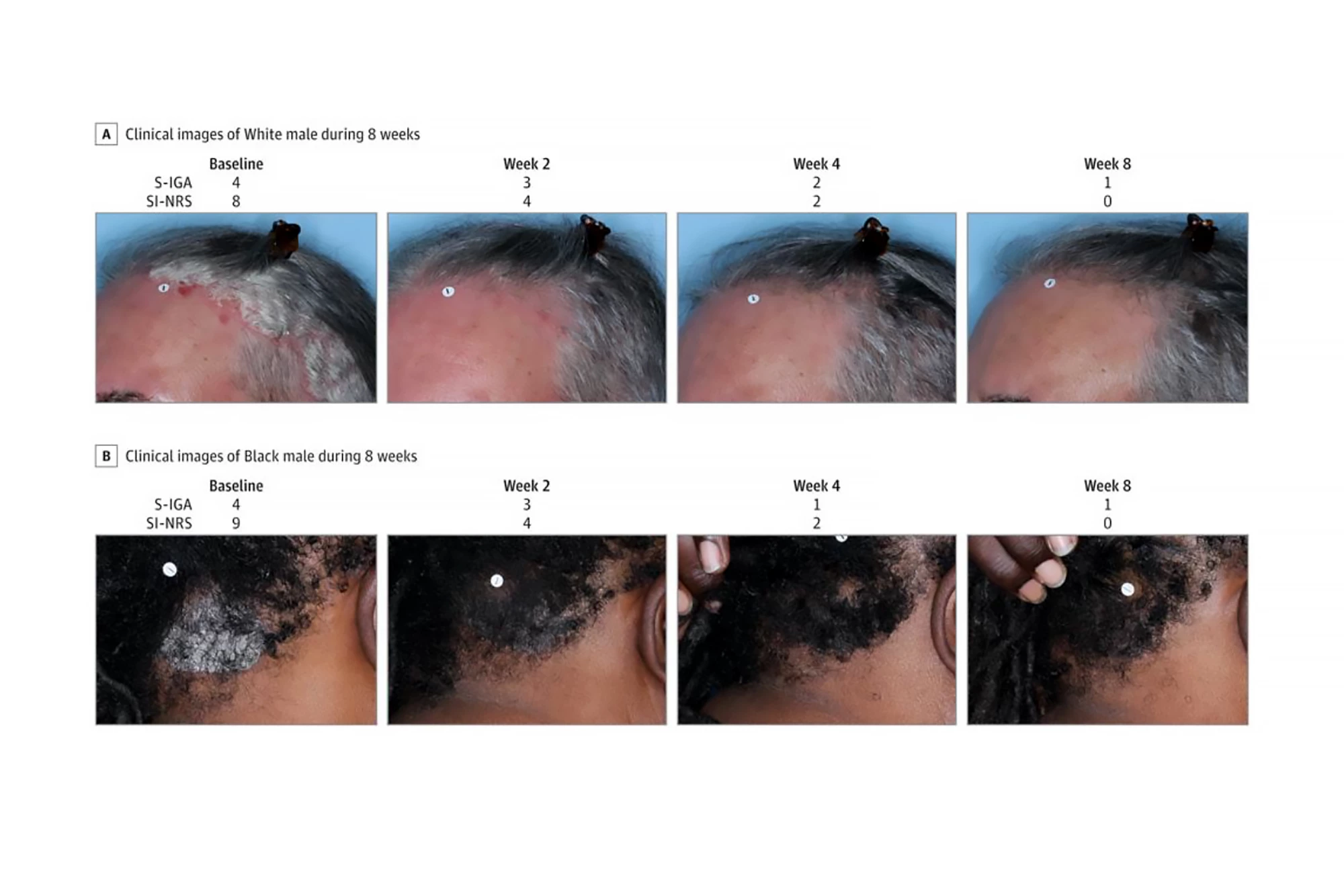
A total of 432 patients (56.3% women) aged 12 and older with psoriasis on the scalp and body were randomly assigned to two groups, one of which used roflumilast foam 0.3% once a day for eight weeks, while the other used an identical foam without the active ingredient (“the vehicle”). Trial investigators measured Scalp-Investigator Global Assessment (S-IGA) and Body-IGA (B-IGA) scores. Both are five-point scales used to assess the severity of skin conditions like psoriasis in clinical trials. They range from a score of zero (clear, no signs of the condition) to four (severe). The primary endpoints were “success” in S-IGA and B-IGA scores, defined as achieving a score of zero or one (almost clear) plus at least a two-grade improvement from baseline at week eight. The investigators also assessed changes in skin itch intensity.
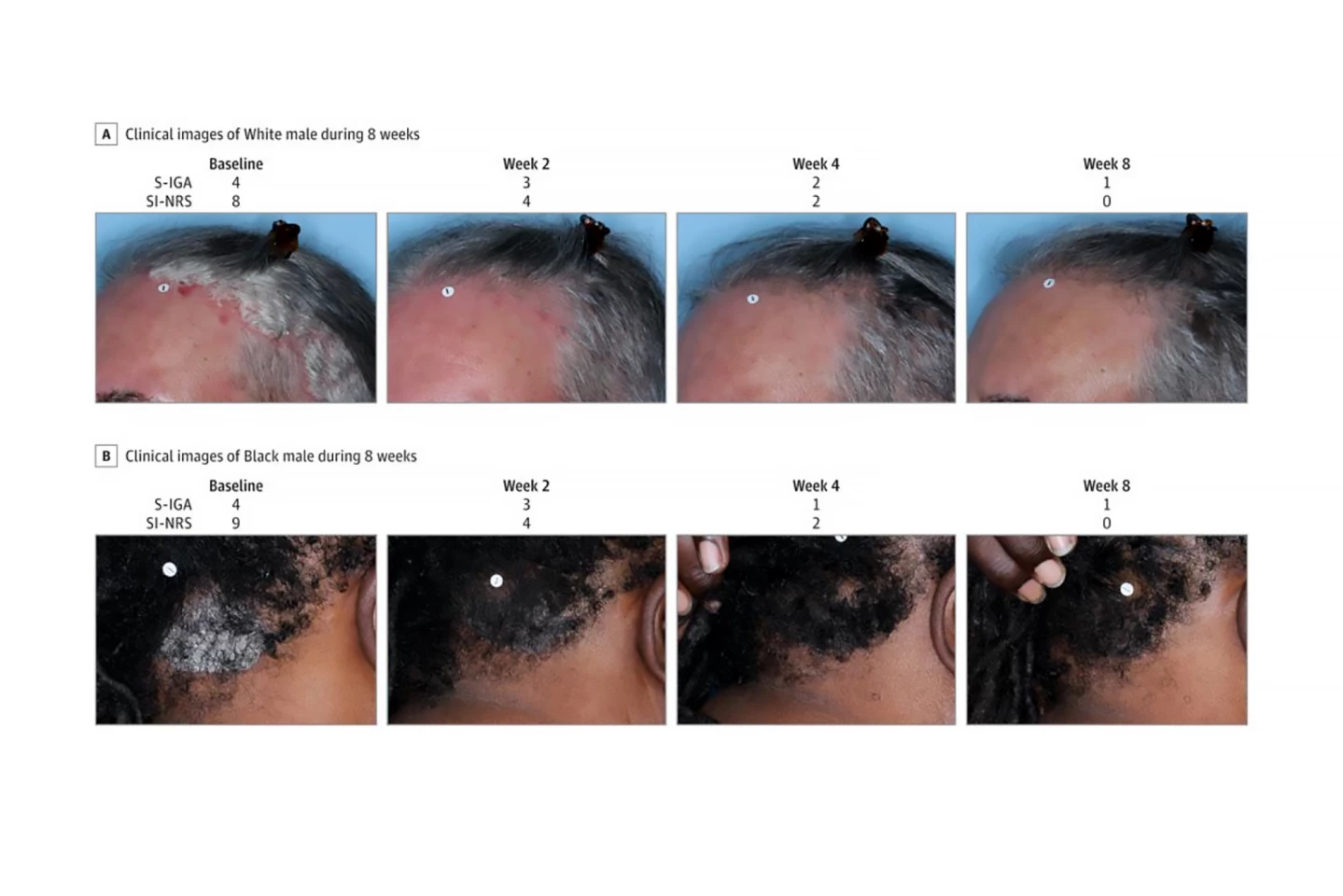
They found that, compared to those who used the vehicle, significantly more patients in the roflumilast group achieved the primary endpoints at week eight: 66.4% achieved S-IGA success compared to 27.8%; 45.5% achieved B-IGA success compared to 20.1%. Additionally, more patients in the roflumilast group achieved an S-IGA of clear (40.0% vs 9.1%) and a B-IGA of clear (27.8% vs 11.0%) at week eight. Improvements in itching severity at weeks two, four, and eight were significantly higher for roflumilast than for the vehicle group. Indeed, improvements in scalp itch, particularly, were seen in the roflumilast group as early as 24 hours after the first treatment.
Low rates of adverse events were observed. The most common adverse event that occurred during the course of treatment with roflumilast, treatment-emergent rather than treatment-related, was headache (4.6%), followed by diarrhea (3.2%) and nausea (2.1%).
“Use of roflumilast foam was associated with significant improvements in both signs and symptoms of psoriasis of the scalp and body, consistent with outcomes previously observed with roflumilast cream, 0.3%, in patients with psoriasis,” said the researchers.
Although it’s a minor limitation, the length of the trial means that the results are limited to an eight-week timeframe. Nonetheless, the significance of the results means that patients with psoriasis of the scalp and body might want to consult their treating medical practitioner to discuss an off-label prescription for roflumilast foam.
The study was published in the journal JAMA Dermatology.