With lithium-ion batteries serving as the engine room for so much of the modern world, from phones and laptops, to electric cars and planes, every scientific breakthrough that improves their performance is an important one. Some of these come from incremental advances that experiment with alternative materials, for example, while some come from re-imagining the whole device and the way they work from the ground up. 2021 produced a stellar crop of discoveries that resulted from researchers thinking outside the box in this way. Let's take a look at the most creative and interesting examples.
Opening up to faster charging

One of the ways scientists hope to improve the charging rates of batteries is by using porous structures for the anode, one of its two electrodes. This offers a greater contact area with the liquid electrolyte that transports lithium ions and enables them to diffuse more easily through the material, potentially making for batteries that charge much, much faster.
In November, we looked at a promising new take on this technology, with scientists at the University of Twente fashioning an anode out of a material called nickel niobate. This featured an "open and regular" crystal structure with identical, repeating channels, making it ideal for ion transport.
This was worked into a full battery cell, with the scientists finding it offered ultra-fast charging rates, 10 times faster than today's lithium-ion batteries. This was a marked improvement on the porous materials proposed so far in this area, which feature disorganized and random channels that cause the structures to cave in during charging and the battery to fail. As a sweetener, the researchers point out that nickel niobate has a higher volumetric density than the graphite used for today's anodes, which could also lead to commercial batteries that are lighter and more compact.
Bringing lithium back from the dead
When a battery is cycled, lithium ions travel back and forth between the two electrodes, but not all of them complete the journey all of the time. This causes electrochemically inactive "islands" of lithium to form in between that remain disconnected from the electrodes, with these clumps causing a decline in the device's storage capacity or even causing it to catch fire.
In an interesting advance this week, scientists at Stanford University figured out a way to not just neutralize these damaging clumps of dead lithium, but bring them back to life to boost the performance of the battery. The team found that by adding a high-current voltage during recharging spurred this inactive lithium into action, causing it to creep "like a worm" and reconnect with the electrode, increasing the battery's lifespan by 30 percent.
According to the team, this breakthrough could lead to improved designs for fast-charging batteries or rechargeable batteries with greater capacities and lifespans. Interestingly, they note that the dead lithium island problem is a real issue for next-generation lithium-metal batteries, which have the potential to hold up to 10 times more energy, so the breakthrough could lead to new solutions that unlock this highly promising architecture.
A battery styled like a BLT
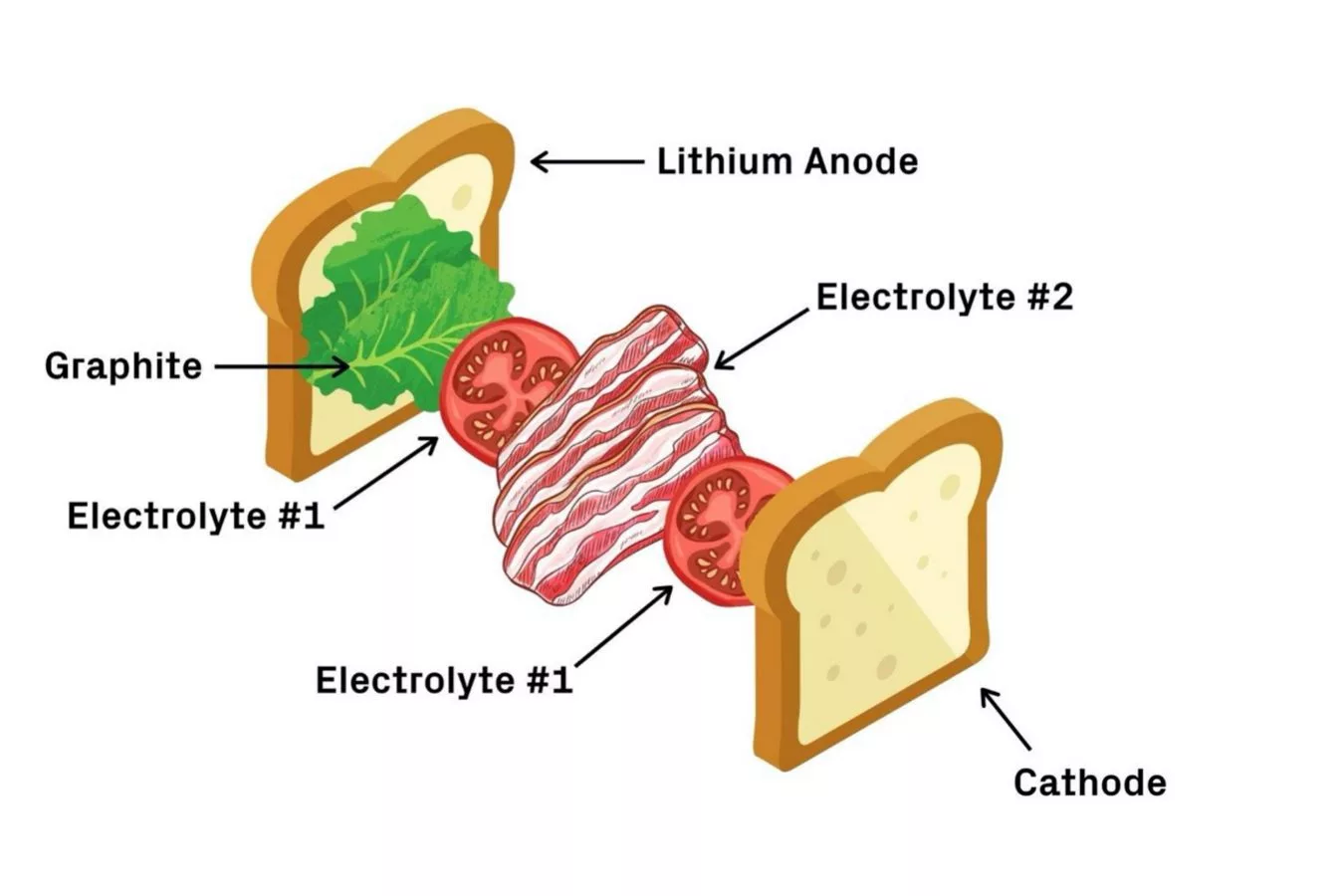
One of the reasons scientists see so much potential in lithium-metal batteries is because lithium metal has a far higher capacity and energy density than the graphite and copper used for the anodes in today's batteries. This positions it as a "holy grail" in the eyes of Harvard material scientist Xin Li, who back in May presented a new sandwich-style battery that could overcome some of the stability issues to plague lithium-metal designs so far.
These stability issues stem from needle-like protrusions called dendrites that form on the lithium-metal anode during charging, causing the battery's performance to decline, and it to fail or even catch fire. Li and his colleagues sought to overcome this by swapping the battery's liquid electrolyte for a pair of solid ones, which are layered together in a BLT-style sandwich and work to safely control and contain the dendrites as they form.
Further, the sandwich-style battery is able to backfill the gaps created by dendrites. In testing, the team found it retained 82 percent of its capacity after 10,000 cycles and, most promisingly, demonstrated the kind of current density that could one day enable electric vehicles to charge within 20 minutes.
Does nature have the answer?

In October we looked at another interesting solution to the stability issues associated with lithium-metal batteries, with a team of scientists in the US turning to nature for inspiration. This breakthrough again hinged on the notion of using a solid electrolyte rather than a liquid one to carry the charge, with the scientists using cellulose nanofibrils derived from wood as their starting point.
These microscopic polymer tubes were combined with copper to form a solid ion conductor, featuring tiny openings in between the polymer chains that acted as "ion superhighways," enabling lithium ions to travel with record efficiency. This meant the material had a conductivity between 10 and 100 times greater than other polymer ion conductors. The researchers also say because the material is paper-thin and flexible, the electrolyte could better tolerate the stresses of battery cycling and withstand the environment of a lithium-metal architecture.
A new take on an old design

Alkali metal-chlorine batteries have been around since the 1970s and offer a high energy density, but the highly reactive chlorine means that they only last for a single use. In August, scientists at Stanford University came up with a way to stabilize these reactions, and actually allow these types of high-density batteries to be recharged.
The solution consisted of a novel electrode material made of porous carbon that sponged up erratic chlorine molecules, and safely converted them back into sodium chloride, their original form prior to discharging. This cycle was able to be repeated up to 200 times in an experimental battery offering around six times the density of today's lithium-ion technology.
Less is more

If it wasn't becoming clear, lithium-metal batteries are a key focus among scientists in this space, and back in June we saw researchers take them into record-breaking terrain. The team focused on what's known as the solid electrolyte interphase (SEI), which is a thin film on top of the anode that plays an important gatekeeping role by controlling which molecules enter from the electrolyte during cycling.
Complex reactions occur around the anode and affect the performance of SEIs in current designs, but scientists at the U.S. Department of Energy’s Pacific Northwest National Laboratory (PNNL) found a novel solution in the form of very thin strips of lithium with a width of around 20 microns, far thinner than a human hair. These were used as the basis for an anode with an SEI that interacts more healthily with the electrolyte than anodes with thicker strips that smother important electrochemical reactions.
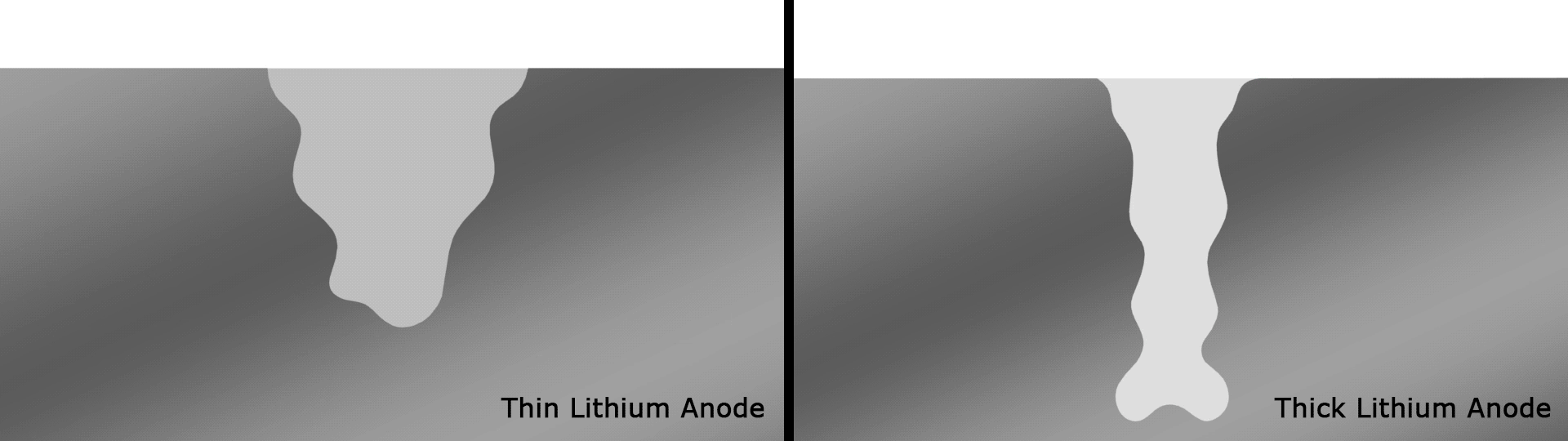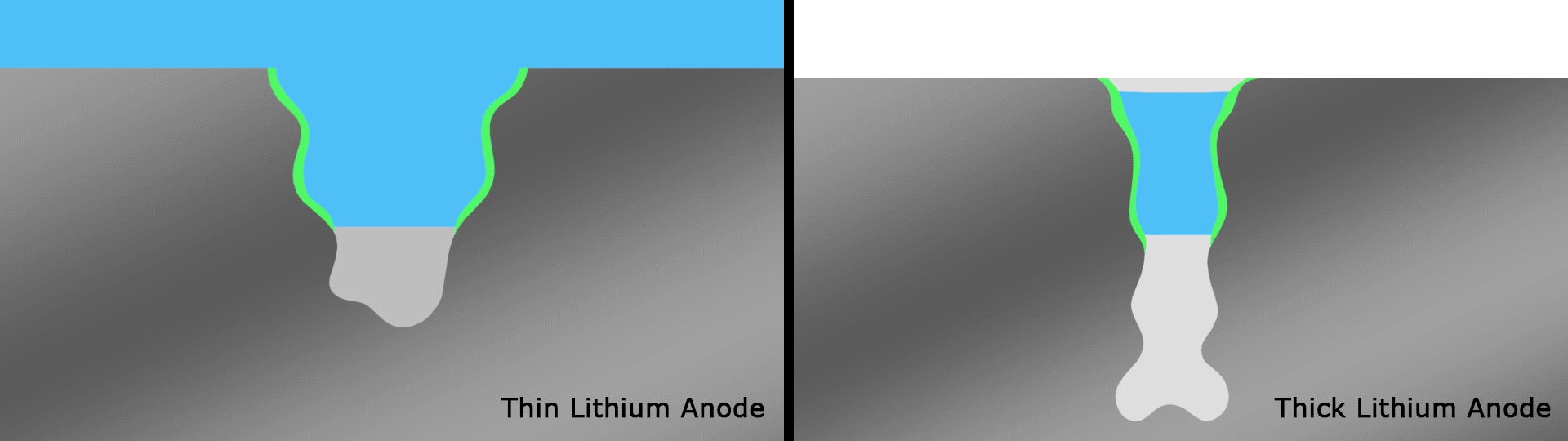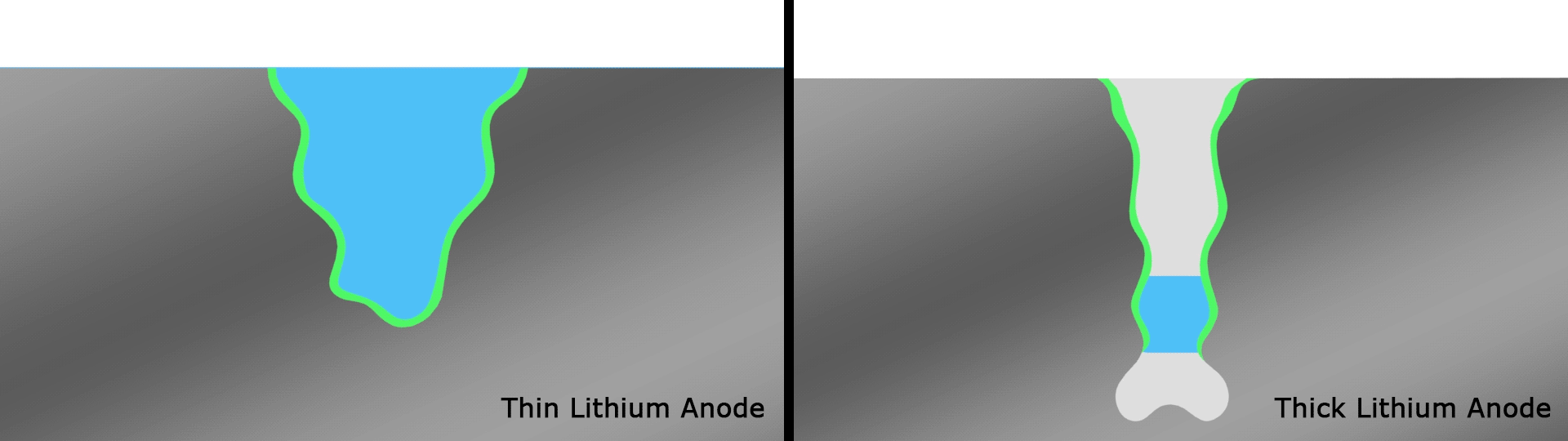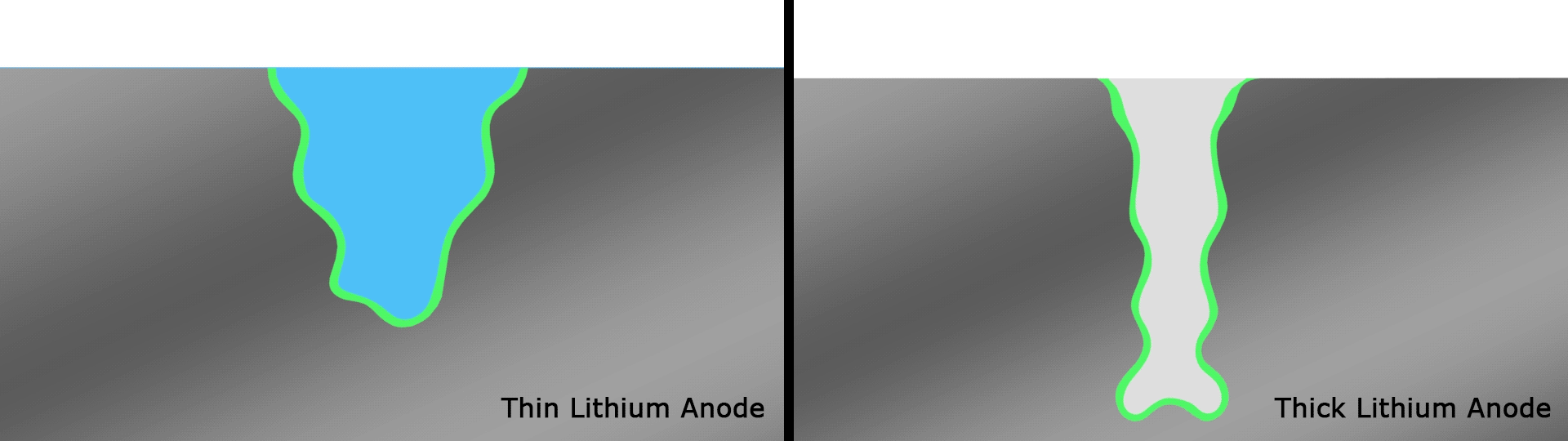
The team's prototype pouch cell battery featuring this anode that retained 76 percent of its capacity over a record 600 cycles, with an energy density of 350 Wh/kg. For reference, the best-in-class lithium-ion batteries in use today have a density of 250 to 300 Wh/kg.
Like filling a cavity
Back in March we looked at another interesting example of a battery that uses a solid electrolyte rather than a liquid one, with the design claimed to overcome some of the key roadblocks in this area. The battery featured a "semi-solid" electrode made of sodium-potassium alloys, likened by the researchers to the material dentists use to fill cavities in that it was firm, but able to flow and be molded.
When this material comes into contact with the solid electrolyte, it has just the right amount of give in it to prevent the type of cracks that would form on a more rigid and brittle electrode material. This self-healing material prevented the formation of damaging dendrites and also allowed for far higher current densities than other solid-state batteries have allowed for – around 20 times greater – paving the way for far greater charging rates.