Australian researchers have developed and tested a way to electrolyze hydrogen straight out of the air, anywhere on Earth, without requiring any other fresh water source. The Direct Air Electrolyzer (DAE) absorbs and converts atmospheric moisture – even down to a "bone-dry" 4% humidity.
Such a machine could be particularly relevant to a country like Australia, which has ambitions as a clean energy exporter, along with enormous solar energy potential – but also widespread drought conditions and limited access to clean water. Decoupling hydrogen production from the need for a water supply could allow green hydrogen to be produced more or less anywhere you can ship it out from – and since water scarcity and solar potential often go hand in hand, this could prove a boon for much of Africa, Asia, India and the Middle East, too.
Chemical engineers at Melbourne University came up with what they describe as a simple design: an electrolyzer with two flat plates acting as anode and cathode. Sandwiched between the two plates is a porous material – melamine sponge, for example, or sintered glass foam. This medium is soaked in a hygroscopic ionic solution – a chemical that can absorb moisture from the air spontaneously.
Hook it up to an energy source, expose it to the air, and hydrogen starts being released at the cathode, and oxygen at the anode, simple as that. The researchers believe this is the first time hydrogen has been pulled directly from the air, and note that it works down to 4% humidity, where even dry areas in Australia's Red Centre, such as Alice Springs, tend to have around 20% humidity.
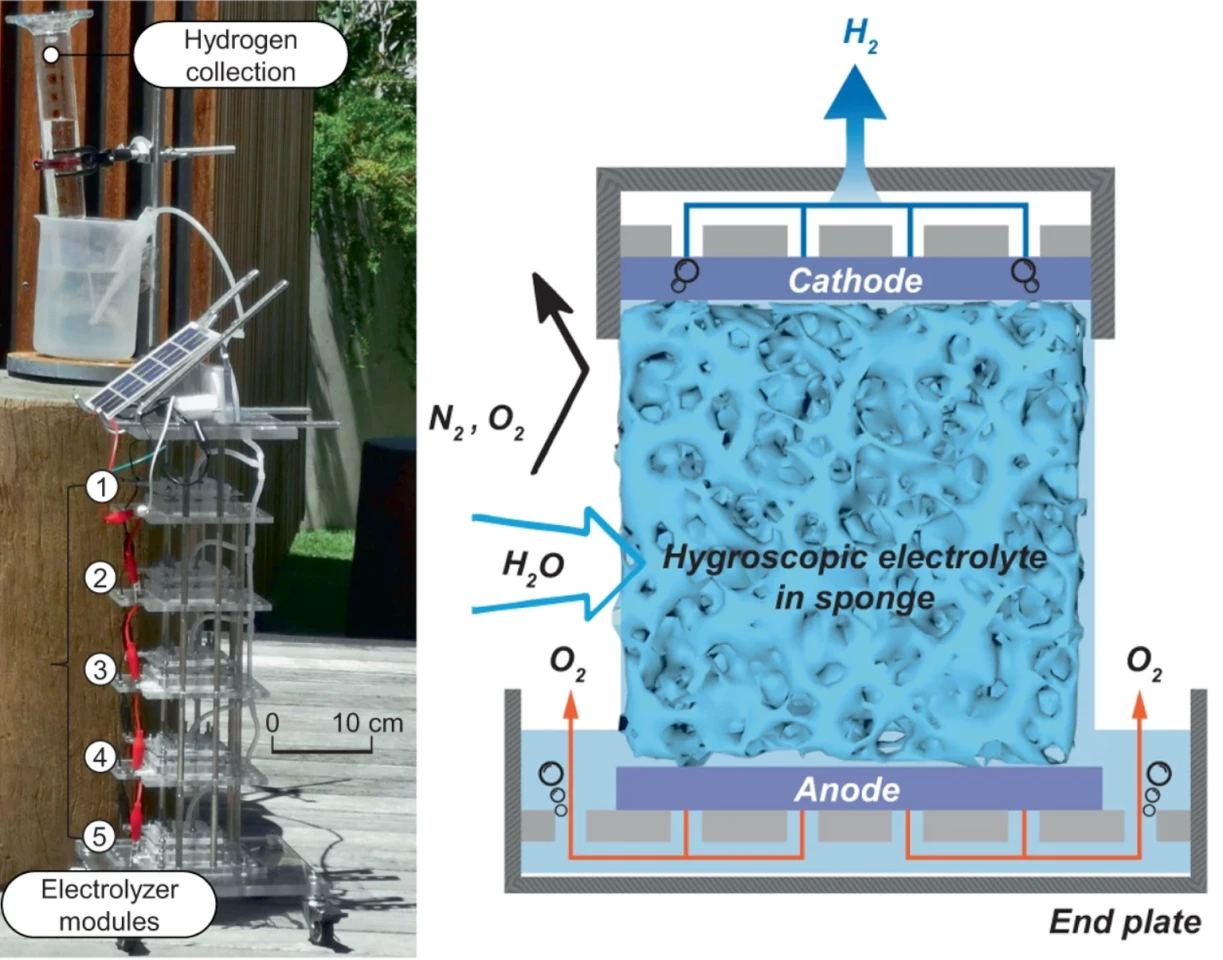
The researchers tested various different hygroscopic liquids, porous media, thicknesses and other parameters, eventually achieving a faradaic efficiency around 95%. Hooked up to a paperback-sized solar panel, the team found the DAE was able to generate 3.7 cubic meters(131 cu ft) of high-purity hydrogen per day, per square meter (10.7 sq ft) of cathode.
The team describes the technology as technically and structurally viable, and low-maintenance, and says the next steps are to test it in a range of harsh conditions and temperatures, and to scale way up.
"We are in the process to scale up the DAE, from a five-layer stack to one meter square, then 10 meters and so on," says Dr. Kevin Gang Li, lead researcher on the paper. "And we can simulate a dry climate in lab, but that's not a real desert. So, we want to take it to Alice Springs and spend a couple of weeks, see how it goes."
The research is open access in the journal Nature Communications.
Source: University of Melbourne






