While hydrogen's high energy per mass makes it an excellent fuel, it's awfully hard and expensive to store long-term. That could change, thanks to the work of researchers at Switzerland's ETH Zurich. They've worked out a way to store hydrogen in ordinary steel-walled containers for months without losing it into the atmosphere – using iron.
Discovering solutions in the past
The research team led by Wendelin Stark, Professor of Functional Materials at ETH Zurich, hit upon this method by drawing from the steam-iron process of producing hydrogen, first invented in 1784.

The group's storage solution is especially suitable in places like Switzerland, where solar power is abundant in the summer, and scarce in the winter.
Surplus solar power is used to split water to produce hydrogen in the summer; it's then streamed into stainless steel reactors filled with iron ore at 752 °F (400 °C). The hydrogen extracts oxygen from the iron oxide, so you're left with iron and water in the reactor, ready to store without expending a lot of energy.
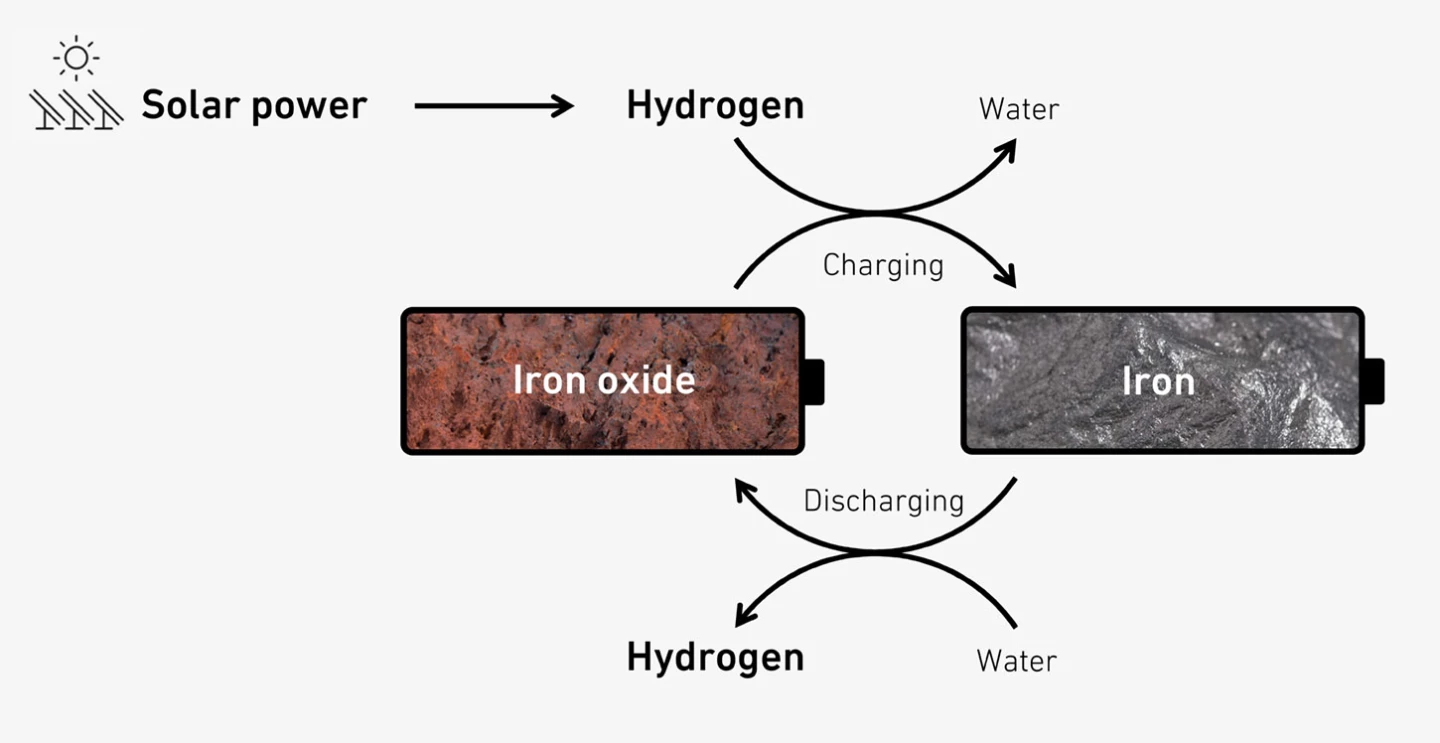
Steam is fed into the reactor to retrieve the stored hydrogen when needed; it can then be converted into electricity or heat easily enough.
There are also several other advantages of using this method:
- The iron ore used in the reactors is cheap, plentiful, and doesn't require processing.
- The reactors themselves are simply made of stainless steel.
- The charging process occurs at ambient pressure – negating the need for high pressure tanks (350-700 bar) typically necessary to store hydrogen gas.
Testing a centuries-old idea
The research team piloted its tech on ETH's Hönggerberg campus, using three stainless steel reactors. Each of them have a capacity of 1.4 cubic meters, and are filled with 2-3 tons of iron ore. The test plant can store about 10 megawatt hours of hydrogen for extended periods, and that'll yield between 4-6 megawatt hours of electrical energy. That's enough to run three to five Swiss homes in the winter. The pilot project is set to grow by 2026, with the team looking to meet one-fifth of the winter electricity needs of the campus using solar power from summer months.

According to the team's research paper published last November, utilizing this system for a single home is currently more expensive than powering it with electricity from the grid. Scaling it up to 100 homes brings the energy cost nearly in line with that of the grid, and it's estimated that it'll only get cheaper as the system expands.
Powering Switzerland and beyond
The researchers note that expanding storage capacity just means adding more reactors, with the processing material serving its charging-discharging cycle duties for years before it needs to be replaced.
In order to provide all of Switzerland with power through the winter months, the team estimates that you'll need about 15–20 TWh of green hydrogen a year, and roughly 10,000,000 cubic meters of iron ore (or 2% of what Australia's iron mines produce).
You'll also need about 10,000 reactor systems, each capable of storing 1GWh. That works out to an area of land equivalent to about 1 square meter per inhabitant of Switzerland.
It's hard to arrive at a clear levelized cost of storage from this small pilot project. And while Switzerland plans to go solar for more than 40% of its electricity needs by 2050, we don't know if it'll invest in hydrogen storage at a national scale. Still, this clever technology from hundreds of years ago seems promising for our seasonal energy requirements in the future.
Source: ETH Zurich





