A nanoporous material that holds hydrogen at twice the density of cryogenic liquid H2 could address the challenges of large-scale liquid and gas storage that have held this clean fuel back.
Hydrogen is finding plenty of applications as a clean fuel – in trucking and commercial vehicles, short range aviation and shipping, for example, where it carries considerably more energy per weight and volume than lithium batteries and can deliver superior range figures and quick refueling. You can burn it more or less like gasoline, or run it through a fuel cell to generate electric power.
It has the highest energy per mass of any fuel, but it's a pain to store. Keep it in gas tanks and you'll need some 700 atmospheres' worth of compression. Keep it as a liquid, and you'll need to maintain cryogenic temperatures just 20 degrees above absolute zero. And even when squashed into a supercooled liquid, it might be lightweight, but it takes up a surprising and inconvenient amount of volume, making it both energy-hungry and tough to package where space is an issue.
Now, Korean researchers say they've created a material that stores hydrogen at double the density of its cryogenic liquid form. “Our innovative material represents a paradigm shift in the realm of hydrogen storage, offering a compelling alternative to traditional approaches,” said Hyunchul Oh, from the Ulsan National Institute of Science and Technology (UNIST), lead author on this new research.
As a molecule, hydrogen can physically adsorb into a porous material in a process called physisorption. Highly porous materials have previously demonstrated the ability to store a large amount of hydrogen per unit mass, but they've struggled to store a lot of energy within a small volume.
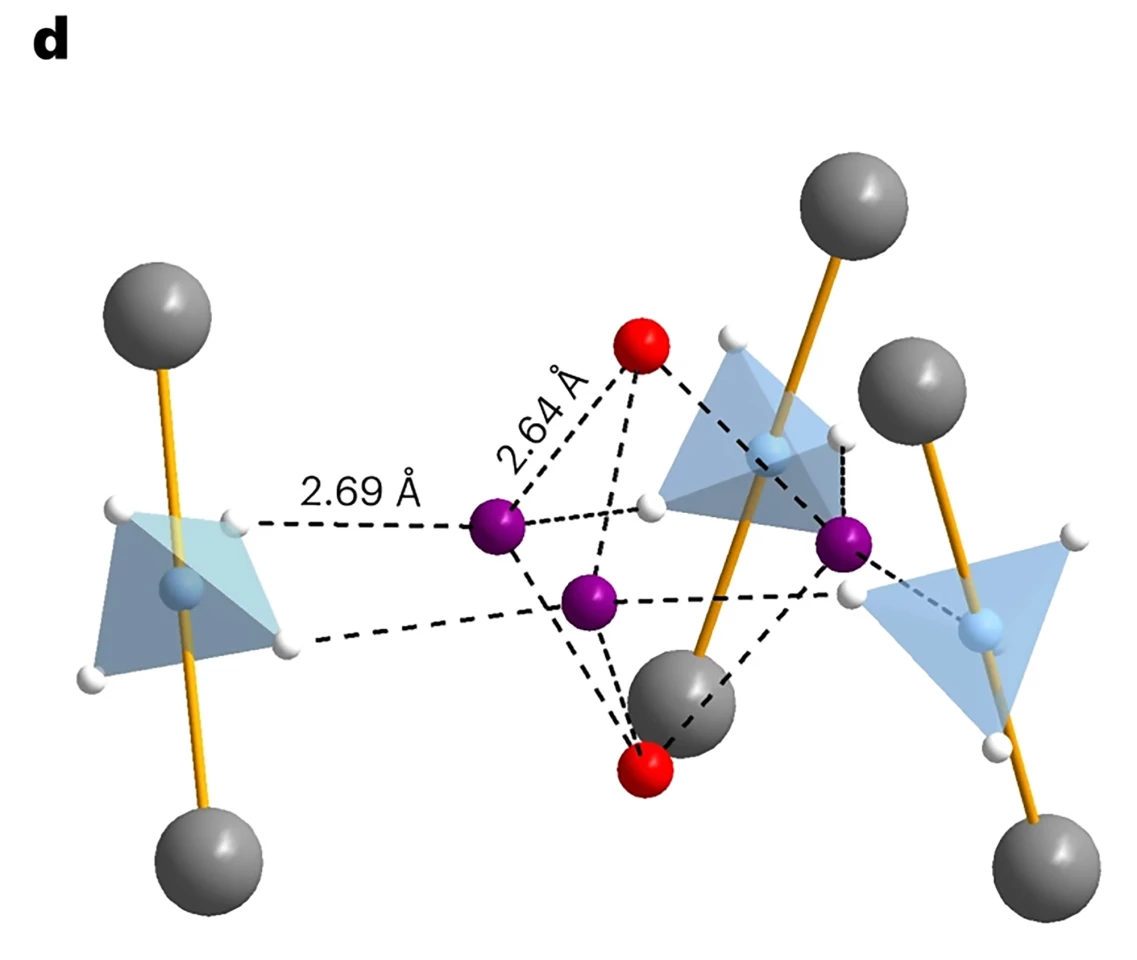
Until now. The team synthesized nanoporous magnesium borohydride (Mg(BH4)2), a framework with partially negatively-charged hydrogen atoms forming the nanopore’s inner surface, enabling the uptake of hydrogen and nitrogen. Although both nitrogen and hydrogen can enter the pores, the researchers found that the gas uptake for hydrogen was larger by a factor of three as both occupy different adsorption sites in the pores.
The high hydrogen density in the small pores, the researchers observed, was due to the anisotropic (direction-dependent) shape of the hydrogen molecules normally seen as close-packed spheroids at near-ambient pressures. The material stored a cluster of five hydrogen molecules in a 3D arrangement, improving volumetric capacity.
They found that Mg(BH4)2 could store an unprecedented 144 g of hydrogen per liter of pore volume, as compared to the 70.8 g/L achieved by cryogenic liquid H2 – or even the 86 g/l you get from solid hydrogen.
The researchers say their findings address critical challenges in large-scale hydrogen storage and enhance the efficiency and economic viability of hydrogen.
Will this be the solution for hydrogen-powered aircraft? Possibly not. As ZeroAvia's Val Miftakhov explained to us several years ago, liquid H2 systems in an aviation setting can achieve a hydrogen mass fraction around 30%, with the other 70% of the weight added by the tanks and cryo-cooling gear. This nanopore storage material, according to the study, delivers a mass fraction of 21.7% – so it carries twice the energy per weight that gaseous H2 in tanks does, but a cryogenic liquid system will be lighter.
On the other hand, it could definitely play a part in long-haul shipping or trucking, where weight is less of an issue and volume is at more of a premium. And certainly, it seems like the best method yet for static energy storage situations, in which hydrogen could be used more or less like a battery.
We'd like to know more about how it's released, what kinds of temperatures and pressures it works under, and what the round-trip energy loss might be of storing hydrogen this way, but it certainly seems like a groundbreaking development in the field.
The study was published in the journal Nature Chemistry.
Source: UNIST






