One problem with renewably-produced hydrogen is that it uses fresh water – and with a quarter of the world's population already facing severe water scarcity at least one month of every year, freshwater is an ever more finite and precious resource. So technologies that can electrolyze hydrogen out of the abundant seawater that blankets most of the planet are a vital area of enquiry.
You can desalinate seawater and then split it, but it's not a great solution; most of your input energy is lost in the desal process, and that drives up the price of the hydrogen you're making. There are also plenty of direct seawater electrolysis machines, but most die too quickly to be useful in a commercial sense; choride ions in the complex ocean brew turn into highly corrosive chlorine gas at the anode, and it eats away the electrodes and degrades the catalysts until the machine stops working.
Researchers at China's Nanjing Tech University believe they've found a way around this problem. In a study published in Nature last month, the Nanjing team demonstrated a direct seawater electrolysis machine that ran for more than 3,200 hours (133 days) without failing. They say it's efficient, scalable and operates much like a freshwater splitter "without a notable increase in operation cost."
The team's electrolyzer keeps the seawater completely separate from the concentrated potassium hydroxide electrolyte and the electrodes using cheap, waterproof, breathable, anti-biofouling, PTFE-based membranes. These membranes stop liquid water from getting through, but they do let water vapor through. The difference in water vapor pressure between the seawater side and the electrolyte side "provides a driving force for spontaneous seawater gasification (evaporation) at the seawater side."
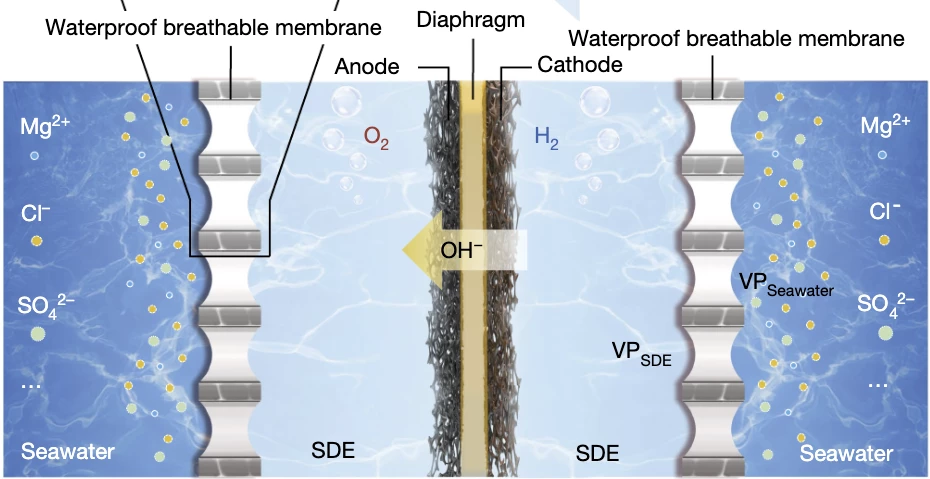
So what you get is pure water evaporating rapidly out of the seawater without any extra energy input, then crossing the PTFE membrane and getting absorbed into the electrolyte as a liquid. According to the Nanjing team, it lets water through and blocks 100% of other ions that might cause damage at the electrodes or the membrane.
The team tested a compact 11-cell electrolyzer box, about the size of a couple of medium-sized suitcases, in seawater from Shenzhen Bay. It generated some 386 liters of hydrogen gas per hour throughout the 133-day test, which sounds like a lot, but if that's at standard atmospheric pressure, 386 liters represents just 31.652 grams' worth of hydrogen. Putting that in a fuel cell EV context and assuming a car drives about 100 km (62 miles) on 1 kg of hydrogen, this 11-cell device generated enough hydrogen per hour to drive a car about 3.2 km (2 miles). Still, it's just a small test unit.
In terms of efficiency, the electrolyzer consumed about 5 kWh for each normal cubic meter (Nm3) of hydrogen produced. Since hydrogen carries around 3.544 kWh of energy per Nm3, this seawater electrolyzer operates at about 71% efficiency. That's certainly in the ballpark of a lot of current electrolyzer tech, although it doesn't keep up with some emerging hyper-efficient designs, such as Hysata' s 95%-efficient capillary-feed design.
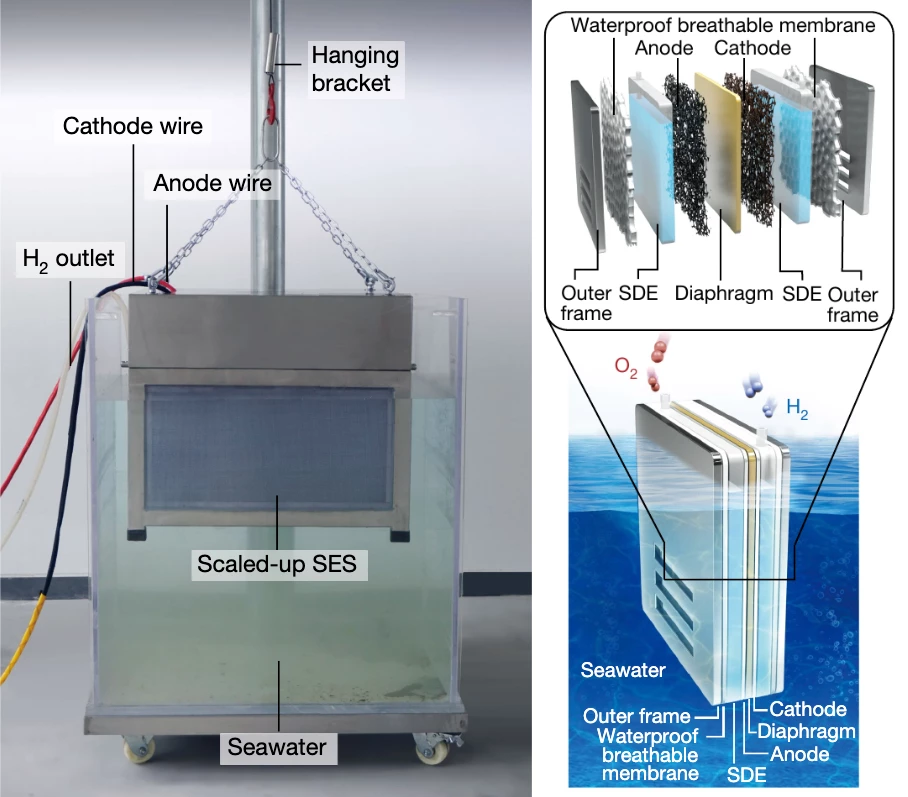
Importantly, the device was still operating at full capacity after four and a bit months in seawater, and post-test analysis showed "no obvious increase in impurity ions" in the electrolyte, "suggesting 100% ion-blocking efficiency" of the PTFE membrane, and there was no corrosion visible on the catalyst layers. The researchers say there are plenty of paths open to explore for performance improvements now that the basic principle for drawing freshwater out of seawater has been proven.
What's more, it could be developed into a lithium-collecting machine as well. Readers with memories better than mine might recall a story we published back in 2020, in which a team from Saudi Arabia's King Abdullah University of Science ant Technology (KAUST) developed and tested a seawater-electrolyzing device that also sucked lithium phosphate out of the seawater using special ceramic membranes.
It's a different system altogether, but the Nanjing team did a little bit of testing to see how their evaporation process affected the concentration of lithium in the seawater. They found a significant 42-fold increase after a couple of hundred hours, and they were able to precipitate out some lithium carbonate crystals, suggesting that with further development, these machines might be able to generate revenue both from hydrogen and battery metals – that could be a huge boost in terms of commercial uptake and scaling.
Very neat stuff. The research is published in the journal Nature.
Source: Nature via IEEE Spectrum






