With the capacity to store up to five times the energy of today's lithium-ion solutions, researchers have a keen interest in lithium-sulfur batteries, and a team at the University of Michigan has taken a step toward realizing their real-world potential. The breakthrough hinges on a naturally inspired membrane that overcomes stability issues and offers the battery a "nearly perfect" design, enabling it to last for a thousand-plus cycles.
“There are a number of reports claiming several hundred cycles for lithium-sulfur batteries, but it is achieved at the expense of other parameters – capacity, charging rate, resilience and safety," said leader of the research team, Nicholas Kotov. "The challenge nowadays is to make a battery that increases the cycling rate from the former 10 cycles to hundreds of cycles and satisfies multiple other requirements including cost."
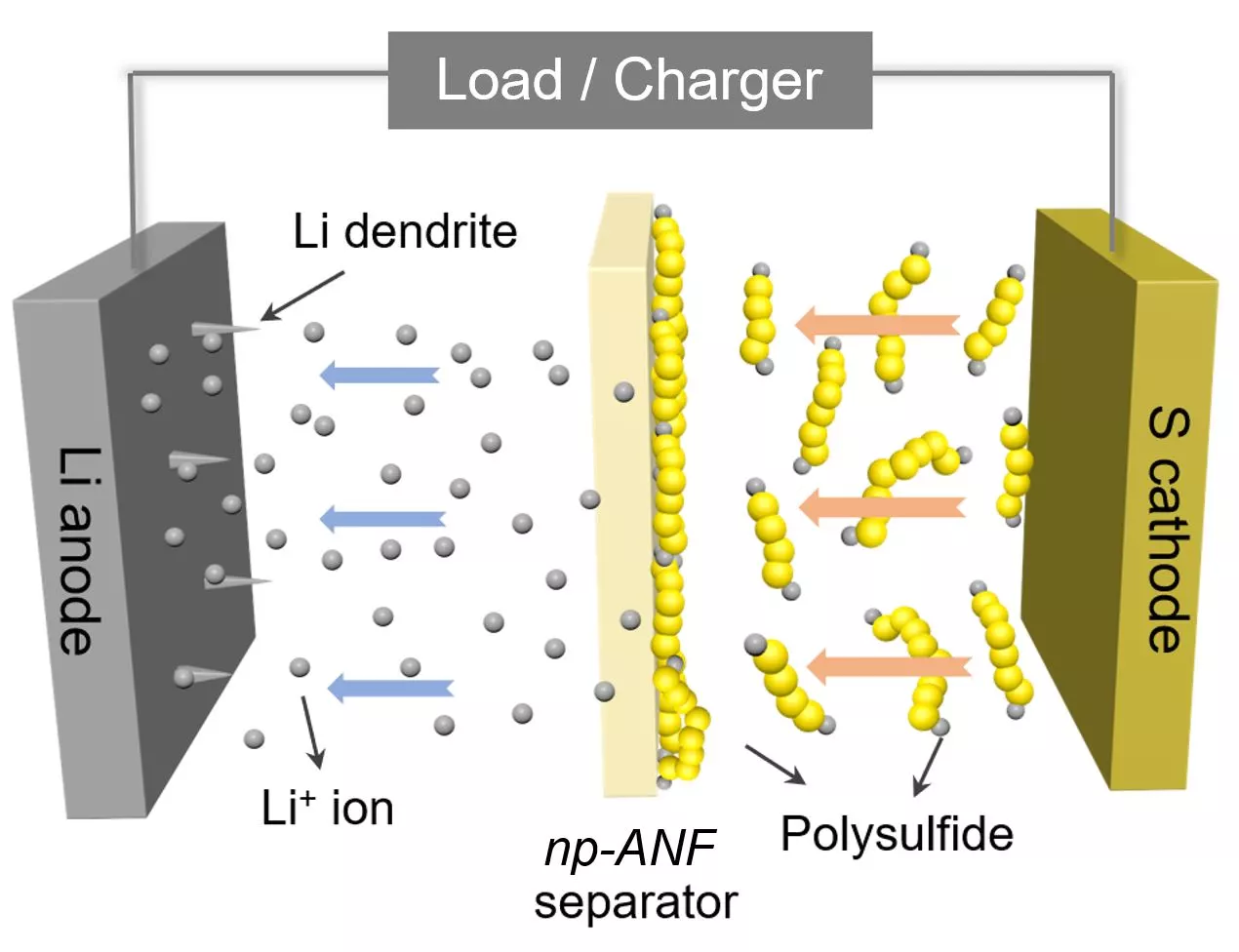
In taking up that challenge, Kotov and his colleagues turned to aramid nanofibers, which are nanoscale versions of Kevlar fibers, and fashioned them into carefully engineered networks that mimic the structure of cell membranes. This material was infused with an electrolyte gel and prevents a common cause of battery failure, which is the formation of needle-like growths called dendrites that grow on one of the electrodes.
But the benefits of the novel membrane go further than that. As a lithium-sulfur battery is cycled, small particles of lithium and sulfur known as lithium polysulfides flow to the lithium and compromise the device's capacity. The team addressed this by integrating tiny, bio-inspired channels into its artificial membrane and adding an electrical charge, which repels the particles while allowing the positively charged lithium ions to flow freely.
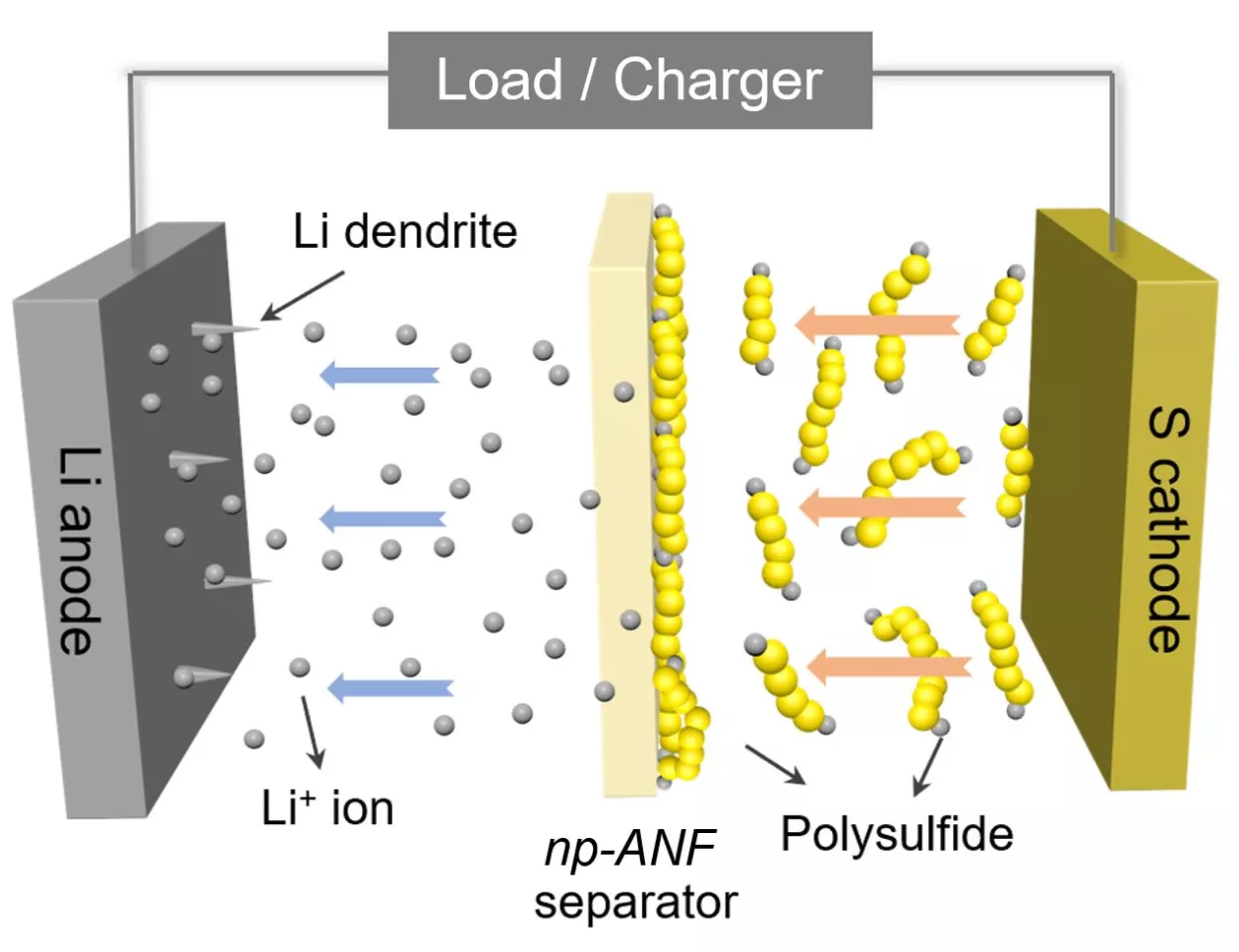
“Inspired by biological ion channels, we engineered highways for lithium ions where lithium polysulfides cannot pass the tolls,” said Ahmet Emre, co-first author of the paper.
The result of this ion selectivity, as it's called, is a lithium sulfur battery with a "nearly perfect" design, according to Kotov. He says the device boasts an efficiency approaching the theoretical limits, while the capacity is five times that of a standard lithium-ion battery. This could one day make for electric vehicles that can travel five times further without needing a recharge, for example.
In a real-world setting with fast-charging technology, the scientists expect the battery would last for around 1,000 cycles, which is considered a 10-year lifespan. Also working in the device's favor is the fact that sulfur is more abundant and less problematic to source than the cobalt used in lithium-ion batteries, while the aramid fibers can be harvested from old bulletproof vests, making it an overall more environmentally friendly proposition.
“Biomimetic engineering of these batteries integrated two scales – molecular and nanoscale," says Kotov. "For the first time, we integrated ionic selectivity of cell membranes and toughness of cartilage. Our integrated system approach enabled us to address the overarching challenges of lithium-sulfur batteries.”
The research was published in the journal Nature Communications.
Source: University of Michigan