Lithium metal batteries are one of the more promising alternatives to the lithium-ion architecture commonly used today, with the potential to hold many times the energy. Material scientists have taken a step toward this future, demonstrating how applying a very specific amount of pressure to a lithium-metal battery during cycling can prevent the formation of tentacle-like growths that would otherwise bring them undone.
The reason lithium-metal batteries hold so much promise is because they seek to use pure lithium metal as the anode material, which can hold as much as 10 times the energy of the graphite used today. The problem holding the technology back, however, is that as the battery is cycled and the lithium ions interact with the anode, they form growths called dendrites on the surface. These protrusions then lead to electric shorts and fires, and swiftly cause the battery to fail.
As such, much research in this area centers on preventing dendrite growth. Some promising advances include self-forming layers that protect the anode, building ultra-thin lithium metal anodes with smoother surfaces, or introducing more stable, solid electrolytes rather than liquid ones to carry the charge between the anode and other electrode, the cathode.
Previous research has also shown that subjecting a lithium-metal battery to pressure during cycling could also hamper dendrite growth, making for neater deposits of lithium particles and improving the stability and lifespan of the device. Scientists at the University of California, San Diego set out to explore this phenomenon, and try to determine what level of pressure could bring about the best outcome.
Their study involved observing the morphology of lithium-metal batteries as they were subjected to different pressures during operation. At lower pressures, the deposited lithium took on a porous and disorderly nature, leaving plenty of room for the dendrites to grow. At a higher pressure of 350 kilo Pascal (around 3.5 atmospheres), however, the lithium was deposited into neat columns without any pores in between, leaving little space for dendrite formation.
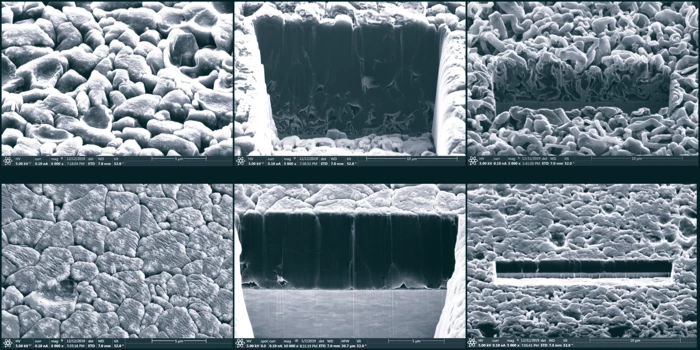
“We not only answered this scientific question, but also identified the optimum pressure needed,” said Shirley Meng, a professor in the UC San Diego Department of NanoEngineering and the paper’s senior author. “We also proposed new testing protocols for maximum LMB (lithium metal battery) performance.”
This kind of manipulation of lithium deposits and the prevention of dendrites will be key in the effort to bring lithium metal batteries to life, but doing so via this high-pressure method would require a rethink on how they are put together. The scientists note that the manufacturing facilities would need to be retooled to produce such a battery, but with these promising results they can now start to explore the possibilities.
The research was published in the journal Nature Energy.




