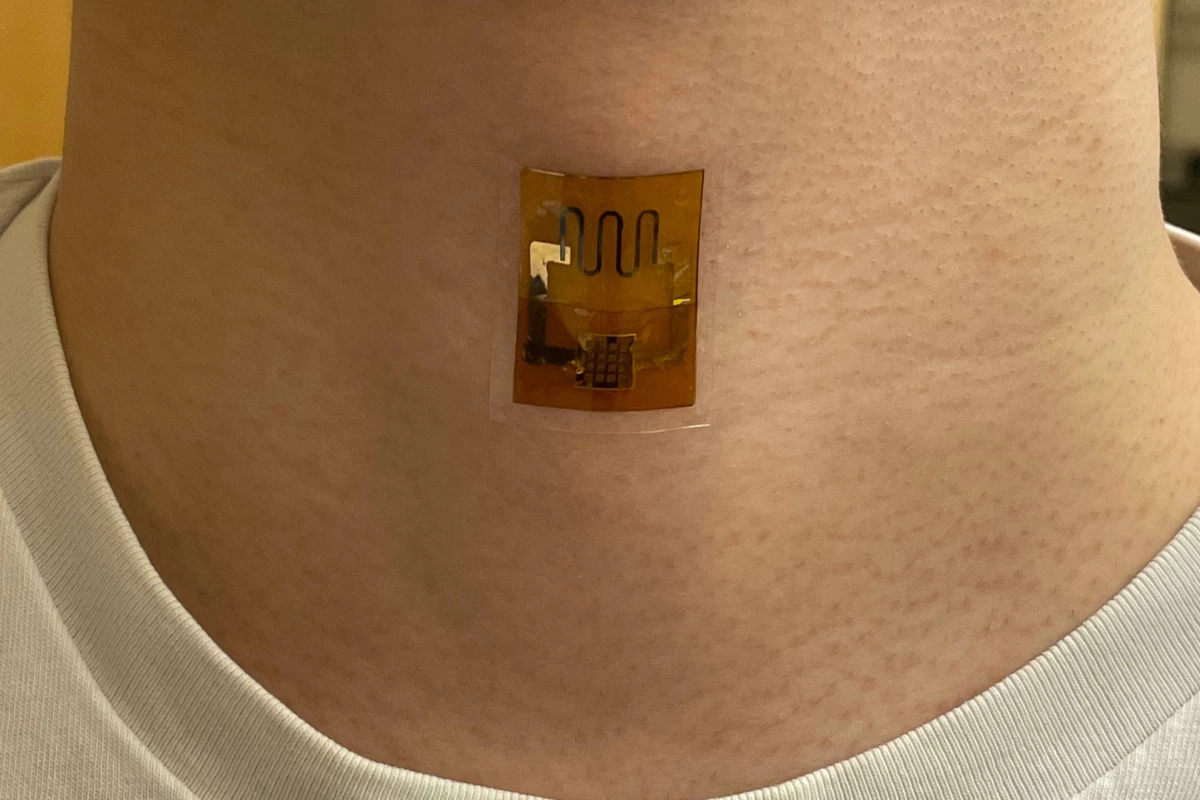
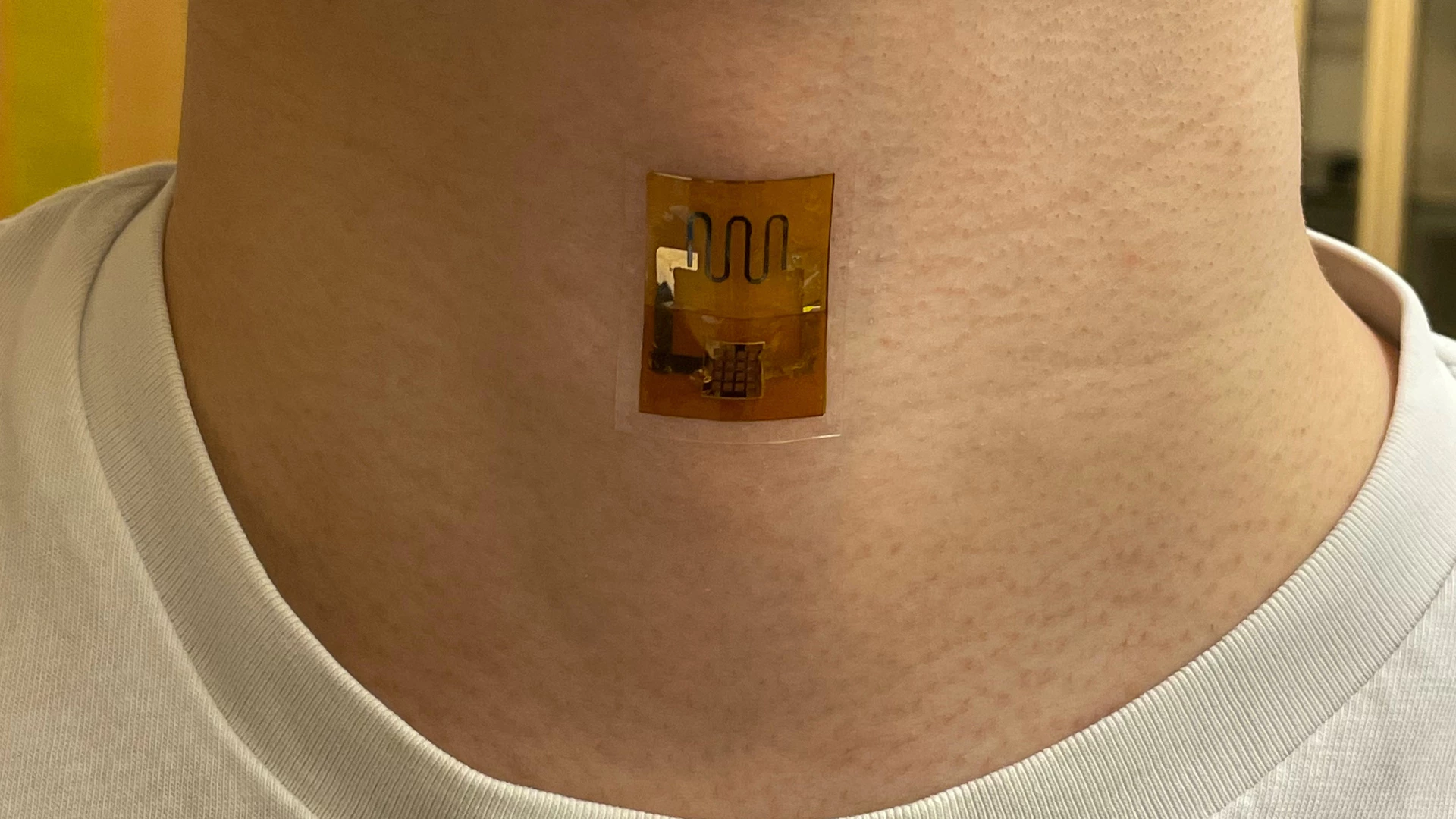
Wearable electronics could soon be powered by dead microbes. New research out of UMass Amherst has demonstrated a biofilm that generates electricity from sweat, harnessing the corpses of dead bacteria – and it's at least as effective as a battery.
The new study, published in Nature Communications, builds on prior research into a particular strain of bacteria known as Geobacter sulfurreducens. Also known as "electricigens," this microbe is one of several that have demonstrated an ability to produce electricity under certain conditions – including during the process of evaporation. That makes it an ideal candidate for use in biofilms, which can be stuck on the skin to harvest usable power for wearable electronics through the evaporation of sweat.
The trouble up until now has been that it's been somewhat of a pain keeping these microbes alive, maintaining suitable conditions and providing continuous feedstocks. The Amherst team discovered that G. sulfurreducens doesn't need to be alive to generate electricity. Indeed, it appears it works even better when freed from the obligations of living.

“It’s much more efficient,” says Derek Lovley, Distinguished Professor of Microbiology at UMass Amherst and one of the paper’s senior authors. “We’ve simplified the process of generating electricity by radically cutting back on the amount of processing needed. We sustainably grow the cells in a biofilm, and then use that agglomeration of cells. This cuts the energy inputs, makes everything simpler and widens the potential applications.”
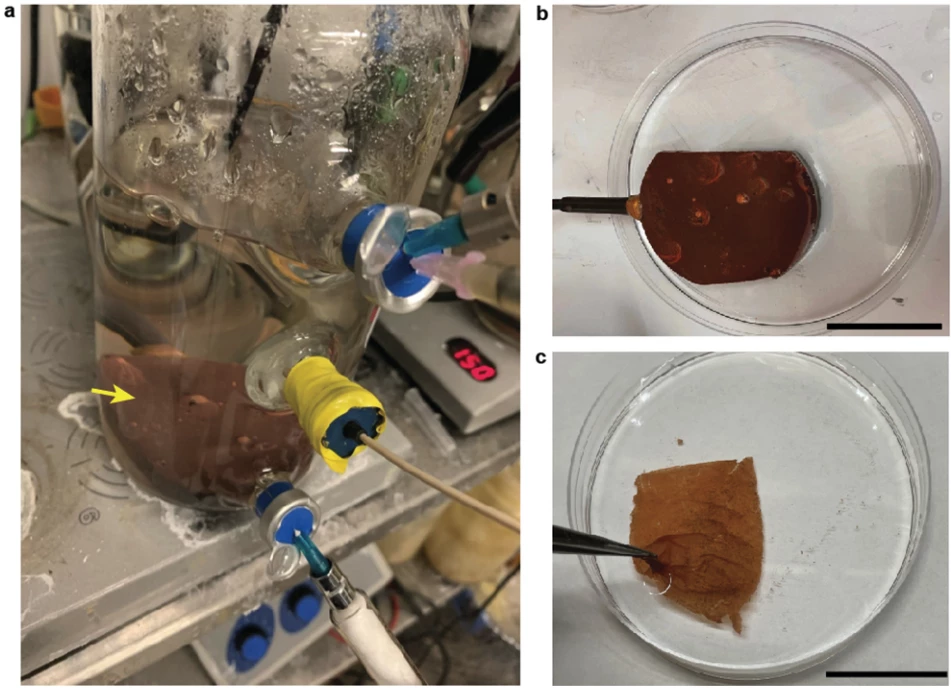
G. sulfurreducens grows in thin, matted colonies about the thickness of a sheet of paper, in which each microbe connects to its neighbors through what the research team calls "natural nanowires." The team takes these mats, and etches small circuits into them using a laser. Then, they're sandwiched between electrodes and encased in a soft, porous polymer patch that's worn on the skin, where it begins generating electricity as sweat evaporates from your skin.
Interestingly, this biofilm appears to work much better than inorganic evaporation-based current generators when it comes to salty water. According to the research paper, its structure also facilitates evaporation – the researchers note that "water evaporation through the biofilms was even faster than from an open water surface."

“The limiting factor of wearable electronics has always been the power supply," says Jun Yao, professor of electrical and computer engineering at UMass, and the paper’s other senior author. "Batteries run down and have to be changed or charged. They are also bulky, heavy, and uncomfortable.” This biofilm, says the team, produces as much energy as a comparably-sized battery, doesn't need to be fed or cared for, and never needs to be plugged in and charged.
The experiments in this paper showed that a biofilm skin patch maintained its performance for at least 18 hours, and capably powered a strain sensor measuring pulse, respiration and other bodily signals. When interconnected, the biofilm sheets powered a laser-patterned wearable electrochemical glucose sensor. In lab testing, the biofilms showed similar performance on the 35th day of testing as they did on the first day.
But the potential here could be much bigger than wearables.
"Our results demonstrate that biofilm sheets are an innovative, sustainably produced material capable of scalable power production from evaporation-based electricity generation," reads the study. "Other strategies for organizing microbial cells into highly channelized, high surface area materials may be feasible. The ubiquity of microorganisms and their proclivity for biofilm formation suggests possibilities for harvesting electricity via similar evaporation-based strategies in diverse environments."
If this proves scalable over large surface areas, well, some 50% of the enormous amount of solar energy that reaches Earth is soaked up in the process of evaporating water, and maybe this technology could represent a way to harness some of that energy – although that's certainly a long way off.
The research is open access in the peer-reviewed journal Nature Communications.
Source: UMass Amherst