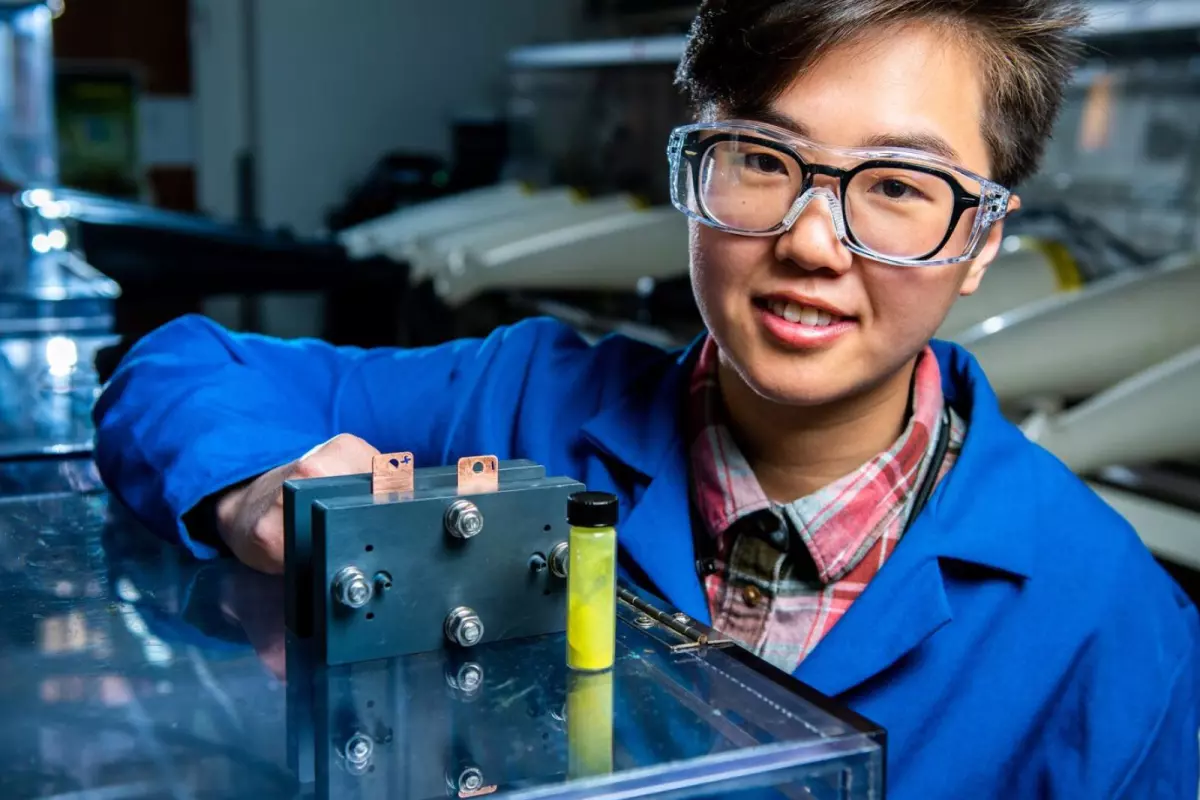
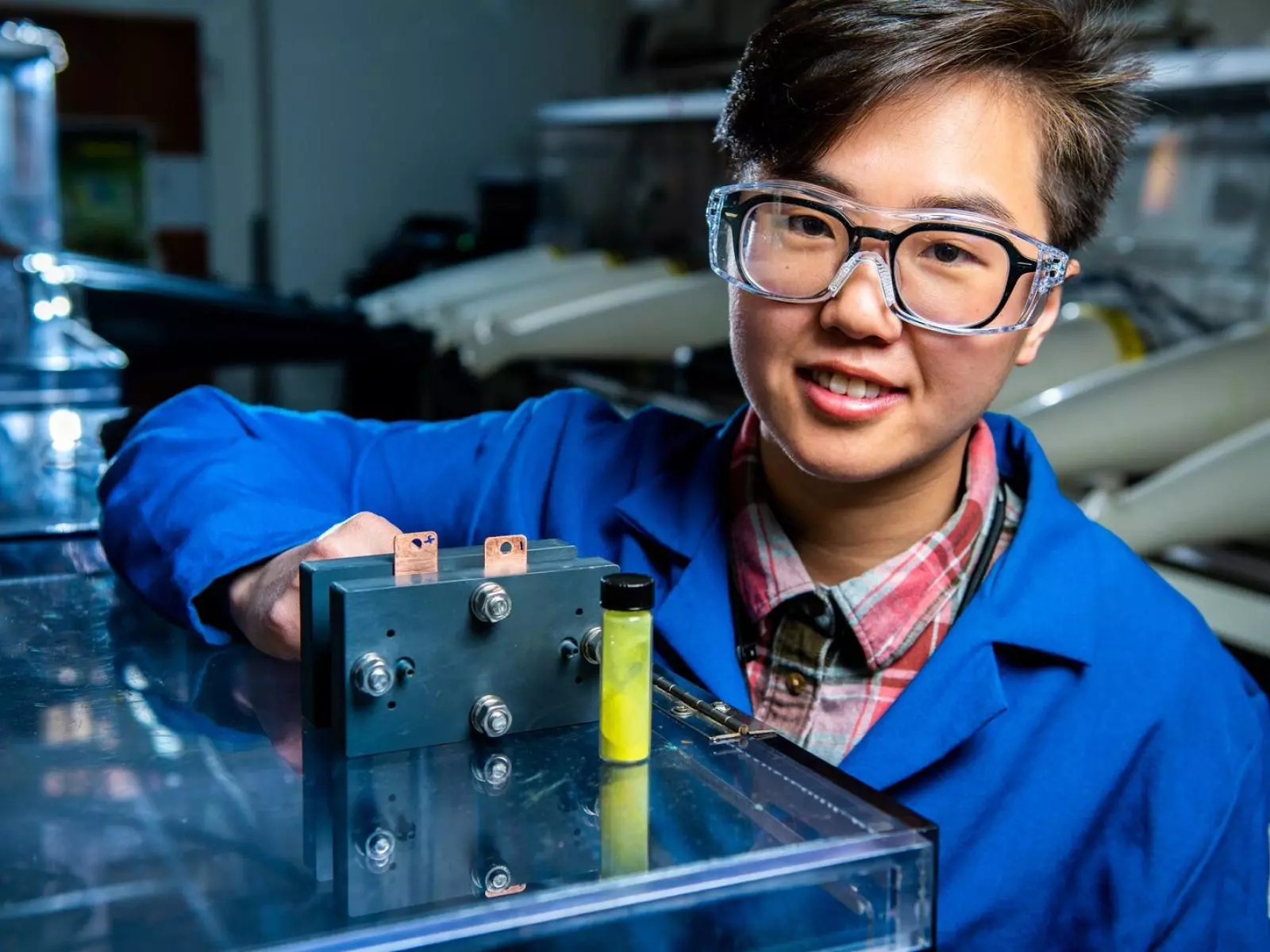
PNNL researchers have discovered that dissolving a simple sugar into the electrolyte in a flow battery boosts peak power output by a remarkable 60%. What's more, after being constantly cycled for a year, the battery lost almost none of its capacity.
Flow batteries – well, most flow batteries, anyway – aren't the kinds of things you'd expect to find in a car or laptop. They're typically best suited to large, long-duration energy storage jobs, so there's been a lot of interest in them in recent years, as cities struggle to work out how to smooth out the daily, seasonal and weather-related intermittency of renewable energy sources.
Typically, they consist of two liquids, an anolyte and a catholyte, that are stored separately, then pumped through on either side of an ion-selective membrane to create a current when energy is needed. This confers certain advantages; you can "refuel" your system by swapping out depleted electrolyte for charged electrolyte, for example – or you can charge it back up using grid power to reverse the discharge cycle.
Pacific Northwest National Laboratory researchers were the first to try dissolving a sugar called β-cyclodextrin into the anolyte of the cell, hoping it would help with a completely different problem. They weren't expecting it to supercharge the battery: “We were looking for a simple way to dissolve more fluorenol in our water-based electrolyte,” said Ruozhu Feng, first author on a new study. “The β-cyclodextrin helped do that, modestly, but it’s real benefit was this surprising catalytic ability.”

Working with Yale researchers, they figured out the mechanism behind the boost – the sugar accepts positively charged protons, balancing out the movement of negatively-charged electrons moving toward and across the cell membrane. After some tuning, they were able to boost the reaction rate, and thus the effective power level of the battery, by a whopping 60%.
These sugars are totally dissolved in the electrolyte, as opposed, for example, to the Influit flow battery technology that's been spun out of Illinois Tech research. Influit uses tiny, solid nanoparticles of active metal oxide battery material, suspended in a viscous fluid – and says its batteries are fast enough to use in cars, with full-system energy densities 4-5 times higher than today's lithium packs.
“This is a brand new approach to developing flow battery electrolyte,” said Wei Wang, long-time PNNL battery researcher and the principal investigator of the study. “We showed that you can use a totally different type of catalyst designed to accelerate the energy conversion. And further, because it is dissolved in the liquid electrolyte it eliminates the possibility of a solid dislodging and fouling the system.”

Once the power output had been optimized, the team moved to durability testing, to see whether the sugar additive would affect the useful life of the electrolyte. The researchers charged and discharged the battery continuously for more than a year, only stopping when a plastic tube broke, and were surprised to find only a negligible loss of capacity.
It's worth noting that flow batteries typically last a lot longer than lithium batteries, but even so, this is the first time, the researchers say, that any flow battery known to published science has ever shown this kind of longevity.
As an institution, PNNL is leaning heavily into solving the grid-level energy storage issue, with a "Grid Storage Launchpad" due to open in 2024. The team has applied for patents for the new technology, and is also starting to trial other, similar compounds that might do the same job even better.
The research is published in the journal Joule.
Source: PNNL