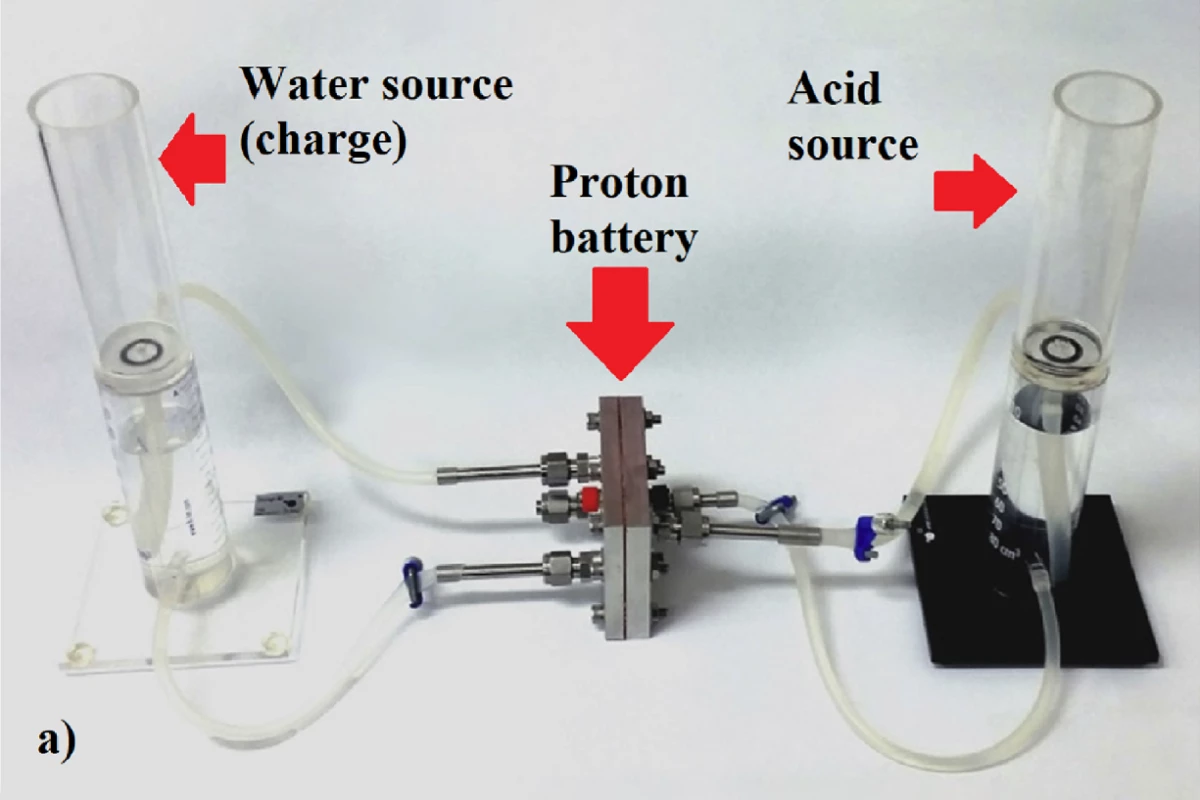
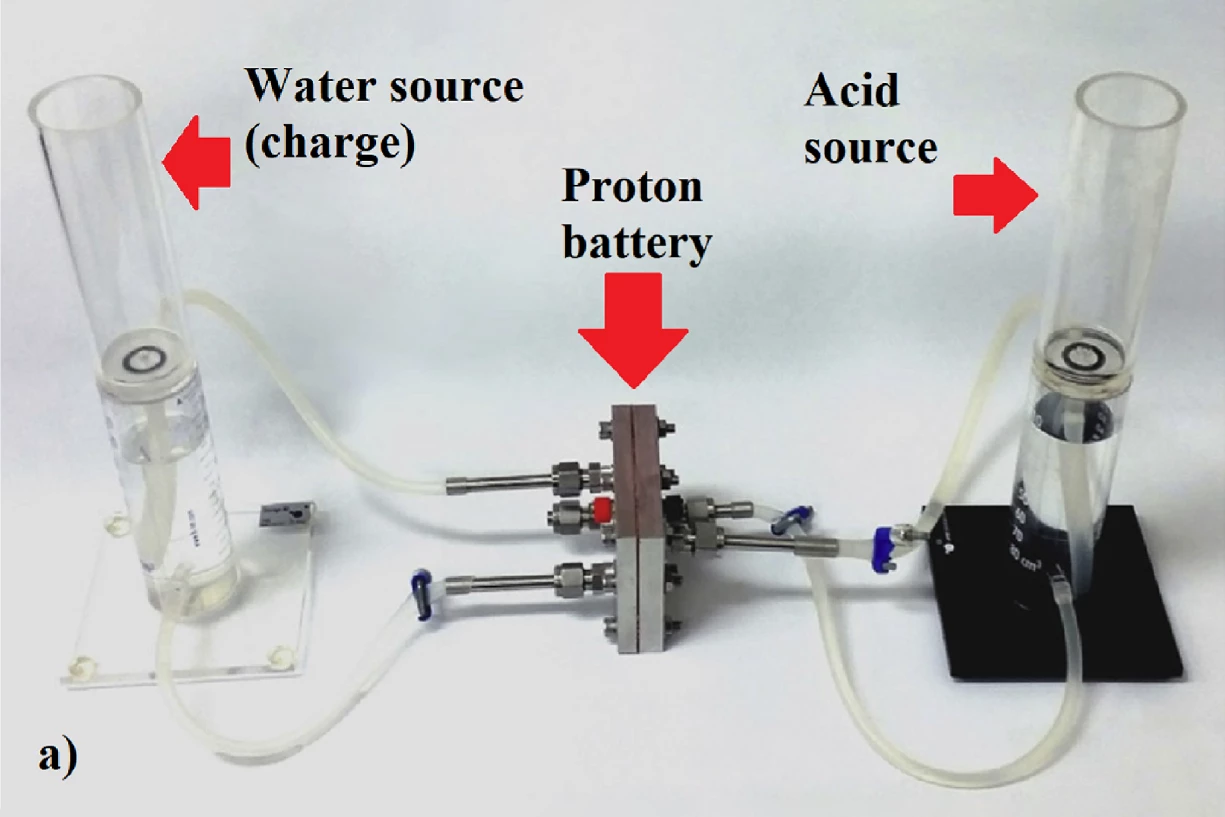
RMIT engineers say they've tripled the energy density of cheap, rechargeable, recyclable proton flow batteries, which can now challenge commercially available lithium-ion batteries for capacity with a specific energy density of 245 Wh/kg.
That's as compared to the ~260-odd Wh/kg delivered by the lithium-ion batteries in a current Tesla Model 3 battery pack, but without using any lithium, thus avoiding a forecasted lithium squeeze, as well as geopolitically sensitive dependence on China in the battery supply chain, and all kinds of end-of-life issues.
We've covered this particular team's work before, way back in 2014, when the first proof of concept of a hydrogen-based proton flow battery was announced.
Essentially, it's a different way of using hydrogen for energy storage. The proton battery works something like a reversible fuel cell, accepting water while charging, splitting out positively-charged hydrogen ions and releasing oxygen.
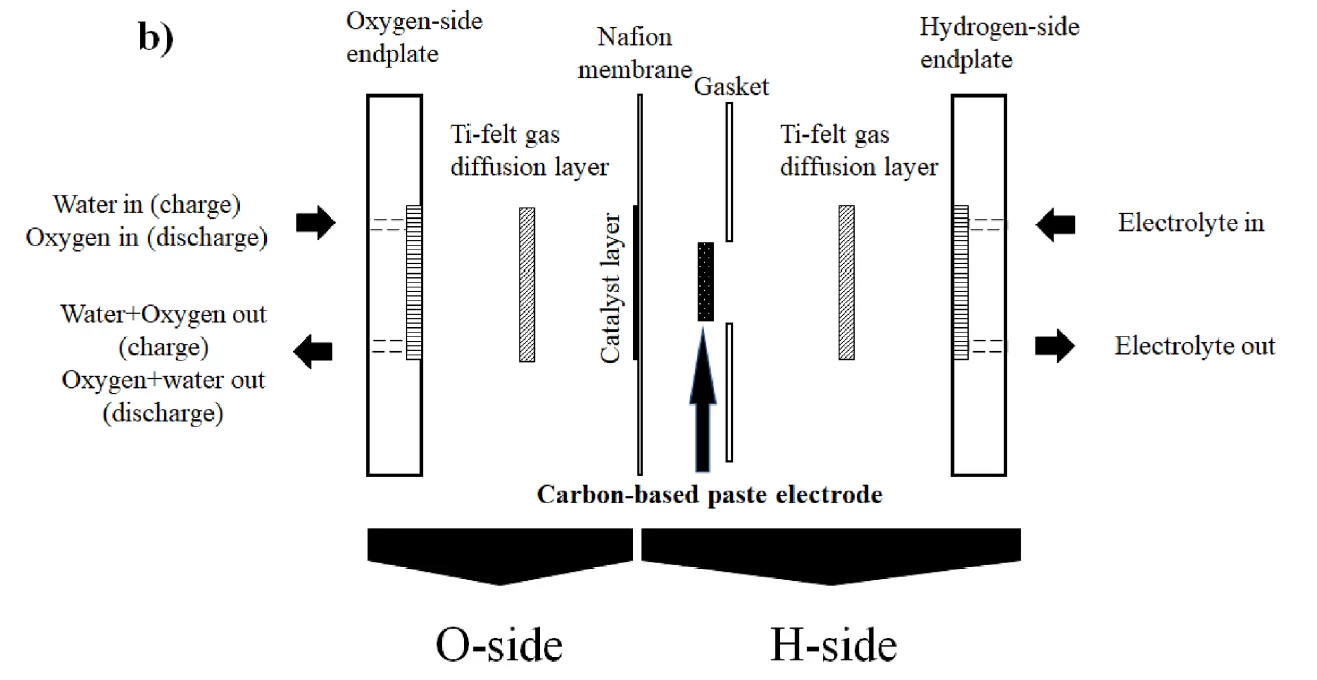
At this point, most hydrogen systems allow these ions to combine into H2 gas, and then expend energy either compressing it, super-cooling it to liquefy it, or further processing it into ammonia. The proton battery instead stores the hydrogen protons directly and immediately, in holes in a solid, porous activated carbon electrode soaked in a dilute acid. Discharging the battery is a matter of adding oxygen, and energy is released as water is produced.
In their latest paper, the RMIT researchers looked into the fundamentals of how the proton battery worked – mainly on the oxygen-side reactions – in order to formulate and test some ideas around how it might be improved. These ideas, according to the paper, included vacuum drying of the activated carbon powder prior to electrode preparation, in order to remove water in the material, mild heating of the overall cell to 70 °C during operation, and replacement of the oxygen-side gas diffusion layer (GDL) with a much thinner GDL fiber sheet.
The benefits, they say, were enormous, resulting in a proton battery capable of storing almost three times as much energy per weight as their last one – and "more than double the highest electrochemical hydrogen storage using an acidic electrolyte previously reported in the literature." At a density of 882 joules per gram, it roughly equates to 245 Wh/kg, right up there with good commercial lithium batteries currently on the market.
So what would be the advantages of a proton battery once it becomes commercially available? Well, it's a very safe and stable way to transport hydrogen, as opposed to high-pressure gas, constantly boiling cryogenic liquid, or highly caustic ammonia. It should last a long time, and be quick to charge.
It'll be relatively cheap, since you don't need lithium or any other exotic metals, and the thing can be made using abundant materials and inexpensive fabrication. It'll also be 100% recyclable.
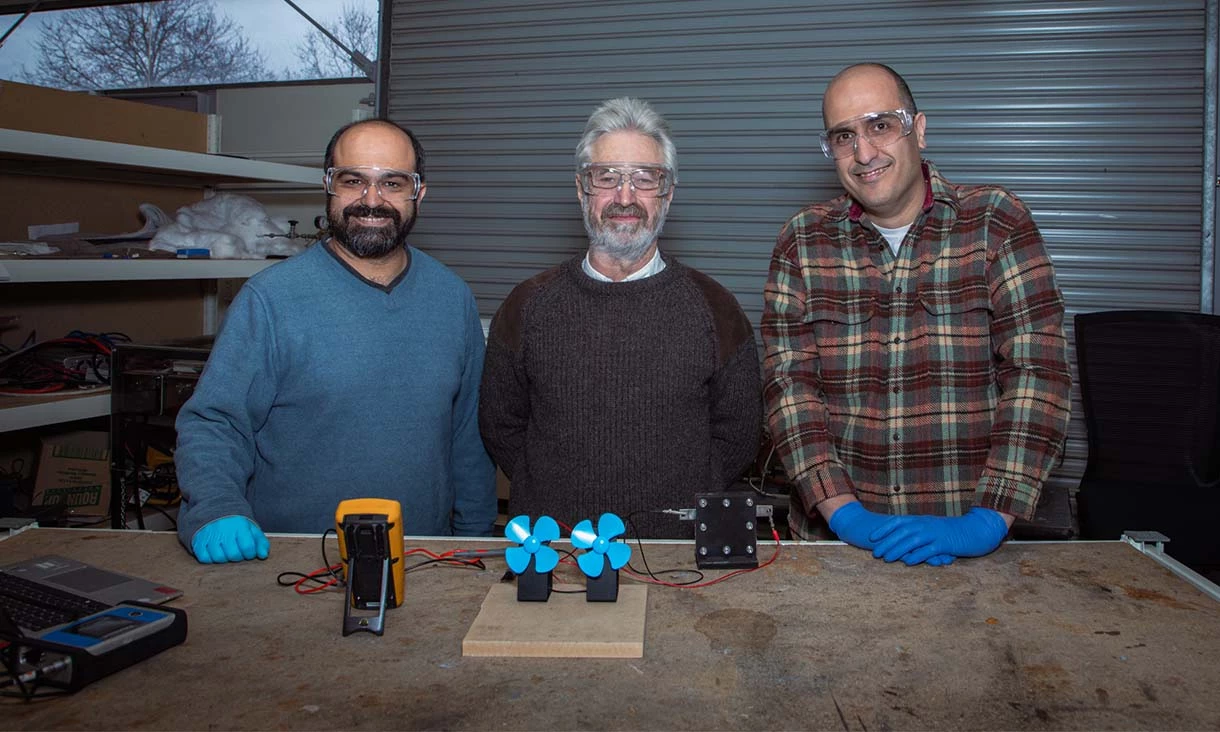
“Our battery has an energy-per-unit mass already comparable with commercially available lithium-ion batteries, while being much safer and better for the planet in terms of taking less resources out of the ground,” said lead researcher and RMIT Professor John Andrews in a press release.
“Our battery is also potentially capable of very fast charging," he continued. "The main resource used in our proton battery is carbon, which is abundant, available in all countries and cheap compared to the resources needed for other types of rechargeable battery such as lithium, cobalt and vanadium. There are also no end-of-life environmental challenges with a proton battery, since all components and materials can be rejuvenated, reused or recycled.”
Roundtrip efficiency is a definite bugbear for most hydrogen powertrains, where energy is effectively thrown away during electrolysis, compression/cooling, storage and at the fuel cell when converting hydrogen back into electricity. But that doesn't seem to be the case here. "Our proton battery has much lower losses than conventional hydrogen systems, making it directly comparable to lithium-ion batteries in terms of energy efficiency" said Andrews.
He clarifies further in an email: "We're targeting above 75% roundtrip energy efficiency at this stage. Yes, this will be time dependent depending on the rate of self-discharge, but we expect this can be minimised with optimal design. As you will know, this is comparable with lithium ion batteries, and much greater than conventional electrolyser/H2 gas storage/fuel cell systems (<45%)."
There's work to be done yet on the overall system design. "The specific energy based on electrode mass quoted in our paper was 245 Wh/kg," he continues, "but this comes down when the mass of other battery components is factored in, although there are many opportunities for keeping these very light. We also expect to raise this figure by optimising overall cell design and operation."
It looks like more of a battery competitor than a fuel cell competitor, though. In applications like aviation where weight is the ultimate priority, gaseous and liquid hydrogen will still carry several times more energy per kilogram of system weight.
Still, the team is moving to commercialize the proton battery. "We are looking forward to developing this technology further in Melbourne and Italy, in partnership with Eldor Corporation, to produce a prototype battery with a storage capacity that meets the needs of a range of domestic and commercial applications," said Andrews. "The aim of this collaboration is to scale up the system from the watt to the kilowatt and ultimately to the megawatt scale."
The research is available in the Journal of Power Sources.
Source: RMIT University
Editor's note: This story was revised on Friday 28th July, to add in some extra comments from Professor Andrews.