Scientists at Harvard University have come up with a promising new design for an experimental battery, one which they say could lead to electric cars that charge in as little as 10 minutes. The alternative architecture is also claimed to offer a far longer lifespan than comparable batteries, by making use of high-performance lithium metal and a solid electrolyte to remain stable over many thousands of cycles.
Scientists hope to improve on the performance of today's lithium-ion batteries by swapping out the graphite and copper used for the anode for lithium metal. The anode works with a liquid electrolyte and the battery's other electrode, the cathode, to pass ions back and forth as the device charges and discharges, and the excellent capacity and density of lithium metal makes it an attractive material for these purposes.
“A lithium-metal battery is considered the holy grail for battery chemistry because of its high capacity and energy density,” says Xin Li, associate professor of materials science at the Harvard John A. Paulson School of Engineering and Applied Science. “But the stability of these batteries has always been poor.”
The reasons behind this poor stability are tiny needle-like protrusions called dendrites, which form on the surface of the lithium metal anode, make their way into the liquid electrolyte and erode the battery's performance, often causing it to short circuit or catch fire.
Using a solid electrolyte instead of a liquid one with its volatile solvents is one of the ways scientists hope to avoid this problem. We've seen promising potential solutions come from research groups at MIT and Australia's Deakin University of late, and now the Harvard scientists are throwing a design likened to a BLT sandwich into the mix.
The team sought to protect its solid-state, lithium metal battery from dendrite-driven destruction by constructing it out of different layers, each with different degrees of stability. These sit in between the two electrodes, which act as the bread, and include a graphite coating (lettuce), a first electrolyte layer (tomato), a second electrolyte layer (bacon), and finally another layer of the first electrolyte (tomato again).
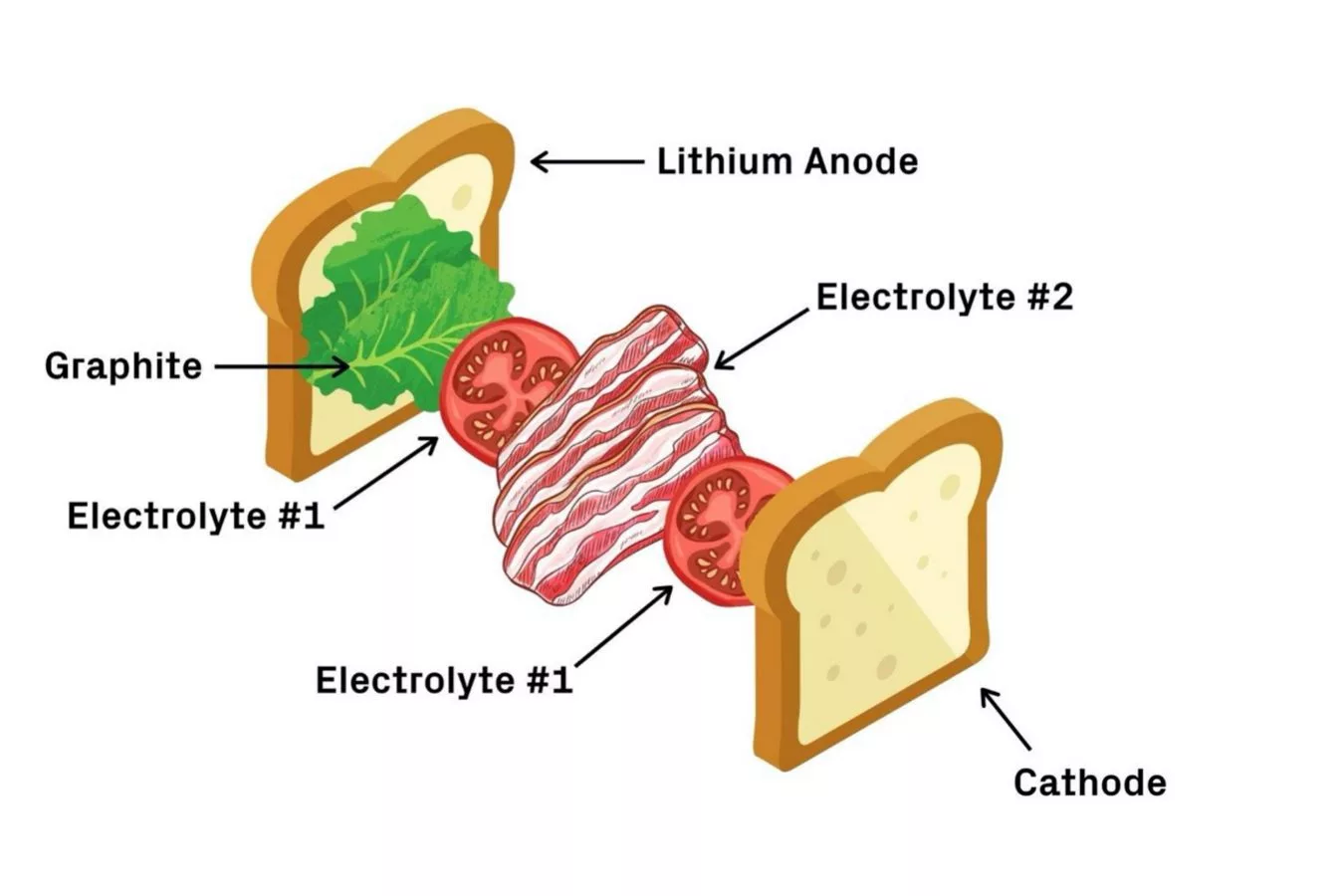
There are slight variations in the chemistry of the two electrolytes, which makes the first prone to dendrite penetration but more stable with lithium, and the second less stable with lithium but immune to dendrites. In this way, the battery doesn't prevent the dendrite formation, but is able to safely control and contain it, with the protrusions being blocked by the "bacon" layer of the sandwich.
“Our strategy of incorporating instability in order to stabilize the battery feels counterintuitive but just like an anchor can guide and control a screw going into a wall, so too can our multilayer design guide and control the growth of dendrites,” says Luhan Ye, co-author of the paper and graduate student at SEAS.
Additionally, the battery is able to heal itself by backfilling the gaps created by the dendrites. In testing, the team found the battery retained 82 percent of its capacity after 10,000 cycles, and demonstrated the kind of current density that could one day allow electric vehicles to charge within 10 to 20 minutes.
“This proof-of-concept design shows that lithium-metal solid-state batteries could be competitive with commercial lithium-ion batteries,” says Li. “And the flexibility and versatility of our multilayer design makes it potentially compatible with mass production procedures in the battery industry. Scaling it up to the commercial battery won’t be easy and there are still some practical challenges, but we believe they will be overcome.”
The research was published in the journal Nature.
Source: Harvard University





