Implanting a pouch of stem-cell-derived pancreas cells under the skin of type 1 diabetics has enabled them to live without insulin injections for years and maintain non-diabetic blood sugar levels, according to the results of a clinical trial. It’s a big step towards a functional cure for the disease.
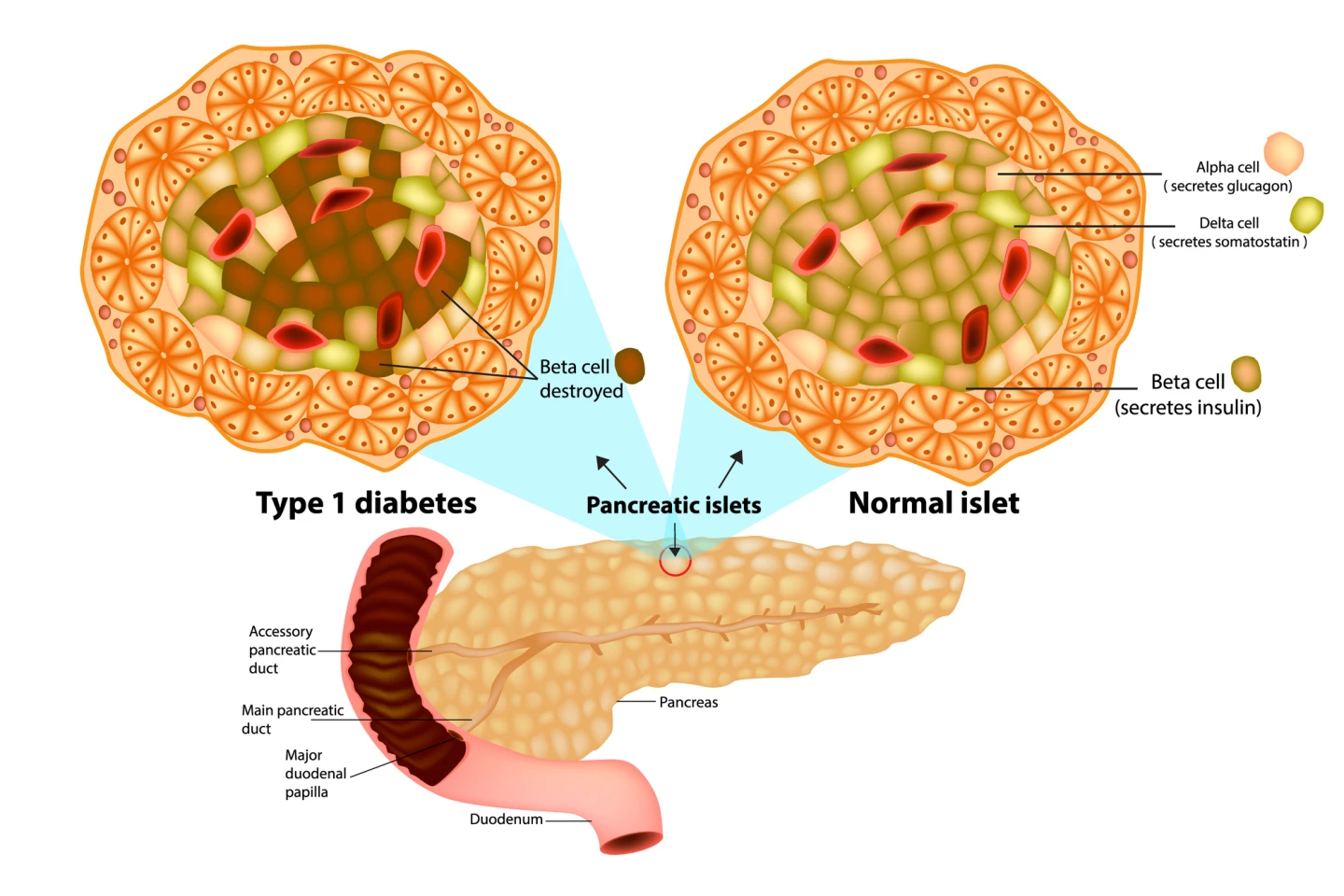
Type 1 diabetes (T1D) is an autoimmune condition where the body’s immune system attacks and destroys the insulin-producing beta cells in the islets of the pancreas, requiring type 1 diabetics to inject insulin daily to replace what’s not being produced. T1D is commonly diagnosed during childhood or early adolescence and requires constant monitoring to reduce the risk of episodes of low blood sugar (hypoglycemia) and long-term complications.
Developing a ‘functional cure’ for T1D has been at the forefront of medical research, especially as science and technology have advanced. Many of these functional cures have involved the transplantation of pancreatic cells to replace the damaged and dead ones. In a similar vein, Canada-based regenerative medicine therapeutics company Sernova Corp has reported very promising results from early clinical trials using its novel Cell Pouch System technology in type 1 diabetics.
“This first-in-world data is potentially game-changing for Sernova and, more specifically, provides tangible hope for T1D patients that we are a significant step further in our mission of providing a functional cure for this terrible disease; as a type 1 diabetic myself, I could not be more determined to drive our program forward and ultimately onto the market,” said Jonathan Rigby, president and CEO of Sernova.
Sernova’s Cell Pouch System is a small, implantable medical device that’s inserted under the skin against the abdominal muscle and contains stem-cell-derived ‘therapeutic cells’ – in the case of type 1 diabetics, cells that produce insulin. Because the device is porous, after implantation blood vessels infiltrate it and form a biocompatible tissue environment that ensures the long-term survival and function of the cells it houses.
About six weeks after implantation of the Cell Pouch, which allows time for patients to be stabilized on immune-suppressing therapy, islet cells are transplanted into the vascularized tissue chambers formed by the pouch. (Immunosuppressants reduce the risk that the patients’ bodies will reject the transplanted cells.) Patients can receive ‘top-up’ islet transplants if they are still dependent on insulin six months after the last transplant. Those trial participants who retained their implants were followed up for at least three years.
The Phase I/II clinical trial of the novel Cell Pouch System recruited 18-to-65-year-old type 1 diabetics who’d had the condition for more than five years and had a history of severe hypoglycemic episodes and hypo unawareness, meaning they couldn’t spot the signs of dangerously low blood sugar. The trial contains two cohorts. Cohort A consisted of six patients who received the first-generation Cell Pouch and Cohort B, seven patients who will receive a Cell Pouch with 50% larger capacity for islets.
All of the six patients in Cohort A achieved sustained freedom from insulin treatment. The first patient to be treated had remained free from insulin injections for more than four years and had blood sugar levels in the non-diabetic range. Their hemoglobin A1c (HbA1c), an indicator of blood sugar control over the past two to three months, was less than or equal to 6.5%. For context, a non-diabetic person’s HbA1c is around 5.7% to 6.4%.
The same patient needed to have the Cell Pouch containing the transplanted cells removed when they developed other health issues, unrelated to diabetes or the device, that required the immune-suppressing medication they were on to be stopped. However, removing the device allowed the trial investigators to examine it under a microscope. Five years after implantation, they found that the tissue was well supplied with blood vessels and consisted of islets of cells that produced insulin, glucagon and somatostatin, all the hormones a healthy pancreas would produce. There were no signs of scar tissue or degradation of the device.
“I am excited to see this evidence of well-vascularized and healthy islets five years after transplant to Sernova’s Cell Pouch; these interim findings are very promising,” said Piotr Witkowski, Director of the Pancreatic and Islet Transplant Program at University of Chicago Medicine and the study’s lead investigator. “This is a major step forward in the development of a contained and retrievable cell therapy for the treatment of T1D. This is the first evidence that I am aware of that demonstrates this level of healthy islet survival and function in an implantable and retrievable system for such a long duration.”
The Cohort B trial is ongoing and the trial investigators should be making its results known soon.
“We look forward to completing Cohort B in the near term and, based on positive data generated thus far, initiating Cohort C of our ongoing trial later this year with an optimized immune suppression regimen,” Rigby said. “Lastly, we continue to work with our partner Evotec on the development of induced pluripotent stem cell (iPSC)-derived islet-like clusters, which will provide a scalable cell source so that one day we can give patients with T1D their lives back.”
Witkowski presented the results of the first cohort of the clinical trial to the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting being held in Madrid, Spain, from the 9th to the 13th of September.
Source: Sernova







