Injecting regulatory T cells or Tregs, which control the body’s immune responses, directly into damaged bone, muscle and skin significantly boosts healing, according to new research. The door is now open to developing a universal cell-based method of enhancing healing after an injury.
A few months ago, we reported on research by the University of Cambridge in the UK that overturned traditional thinking about regulatory T cells or Tregs, finding that these active controllers of the body’s immune response have the potential to be used as an army of healers for “almost everything”.
Now, researchers from the Immunology Frontier Research Center (IFReC) at Osaka University, Japan, and Monash University in Melbourne, Australia, have investigated that potential as part of a new study – and found that it’s true.
“We began exploring administering Tregs for regenerative medicine purposes because they can directly impact other immune cell types called monocytes and macrophages,” said Mikaël Martino, an associate professor at Monash who also held a cross-appointment position at Osaka University, and the study’s corresponding author. “Additionally, Tregs can secrete signaling molecules that support tissue healing. Despite their strong potential, few studies have explored using Tregs for such applications.”
Monocytes are white blood cells responsible for fighting certain infections and helping other white blood cells remove dead or damaged cells. Macrophages, another type of white blood cell of the immune system, engulf and digest (phagocytose) pathogens like microbes, cancer cells, cellular debris and foreign substances.
Essential to healing and tissue restoration post-injury is the body’s ability to transition from a pro-inflammatory to an anti-inflammatory state. There’s plenty of scientific evidence about what can occur when the inflammatory response is not switched off and becomes chronic. Understandably, regenerative medicine therapies seek to capitalize on the main immune system players in this pro- and anti-inflammatory process. That’s where Tregs come in.
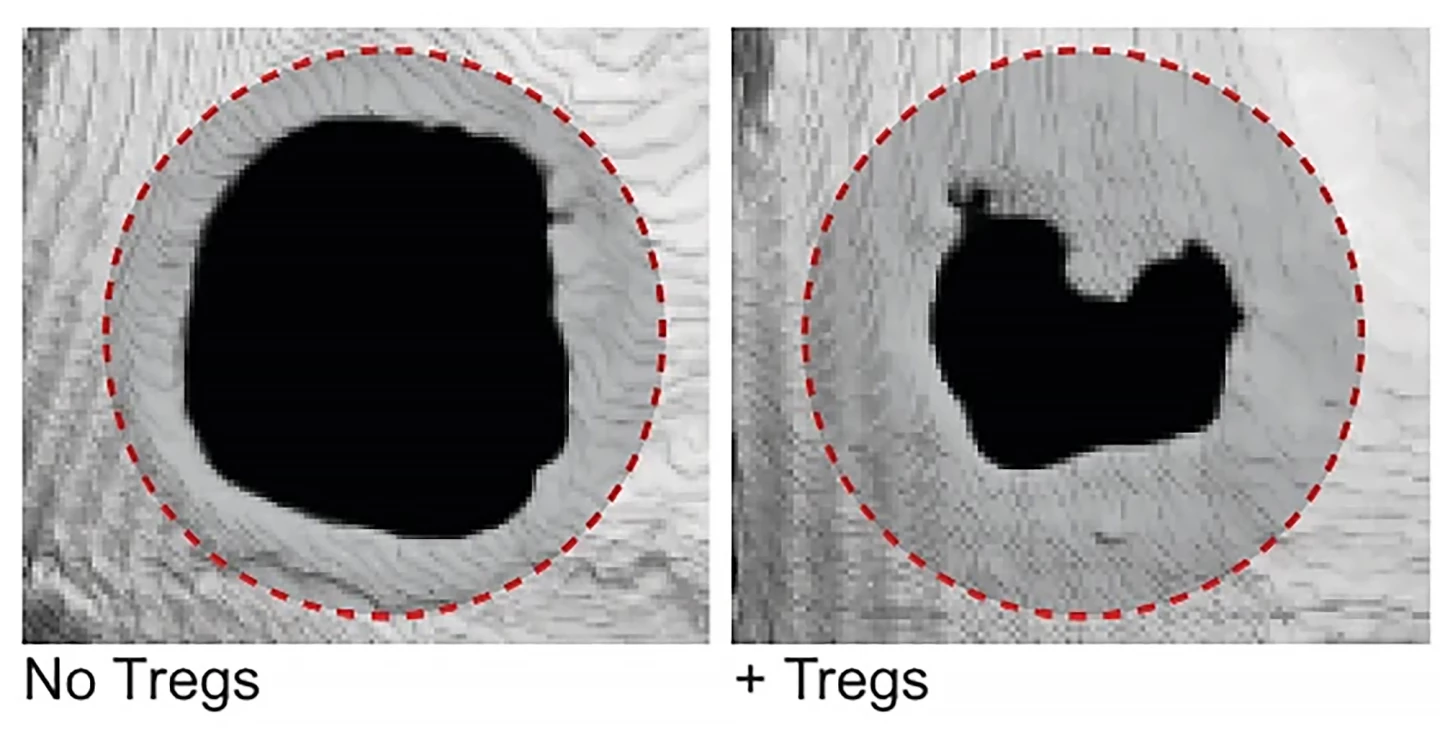
In the present study, the researchers locally administered a fibrin hydrogel containing Tregs into the injured tissue of mice to see to what extent they promoted tissue healing in bone, muscle and skin. Specifically, they chose three models of acute injury: severe skull defects, loss of skeletal muscle resulting in impaired function, and full-thickness skin wounds. Fibrin is a protein that’s naturally involved in wound healing; it’s the end product of the body’s blood clotting pathway and can also act as a medium for regenerative cells like Tregs.
“Compared to those administered fibrin hydrogel without Tregs, mice given Tregs showed enhanced bone volume and coverage over injured cranial areas, higher amounts of muscle tissue and large muscle fiber size, and faster skin wound closure,” said Shizuo Akira, a professor from IFReC and a senior author on the study.
Examining the mechanics of the Treg-promoted healing, the researchers observed that the cells adopted an injury-specific phenotype – a phenotype is an observable trait – after being introduced to the damaged area. The Tregs showed increased levels of expression in genes related to immune system modulation and tissue healing. Further experiments showed that the Tregs caused monocytes and macrophages in damaged tissue to switch to an anti-inflammatory state, specifically by secreting signaling molecules like interleukin-10 (IL-10).
“Interestingly, we observed that when the gene encoding IL-10 is knocked out of the Tregs, their pro-healing effects are lost,” Martino said. “This finding indicates the key role of IL-10 in how these Tregs support tissue repair and regeneration.”
The study’s findings demonstrate the strong potential for using Tregs as a cell-based regenerative medicine therapy after tissue injury. Although this study examined the effect of Tregs administered immediately post-injury, future studies will determine the time frame in which Tregs need to be administered to damaged tissue to effectively aid healing.
The study was published in the journal Nature Communications.
Source: IFReC







