A single injection of a new, off-the-shelf stem cell therapy for knee osteoarthritis significantly improved pain and function for up to 12 months in 75% of participants involved in a clinical trial. The treatment also has the potential to halt the disease’s progression.
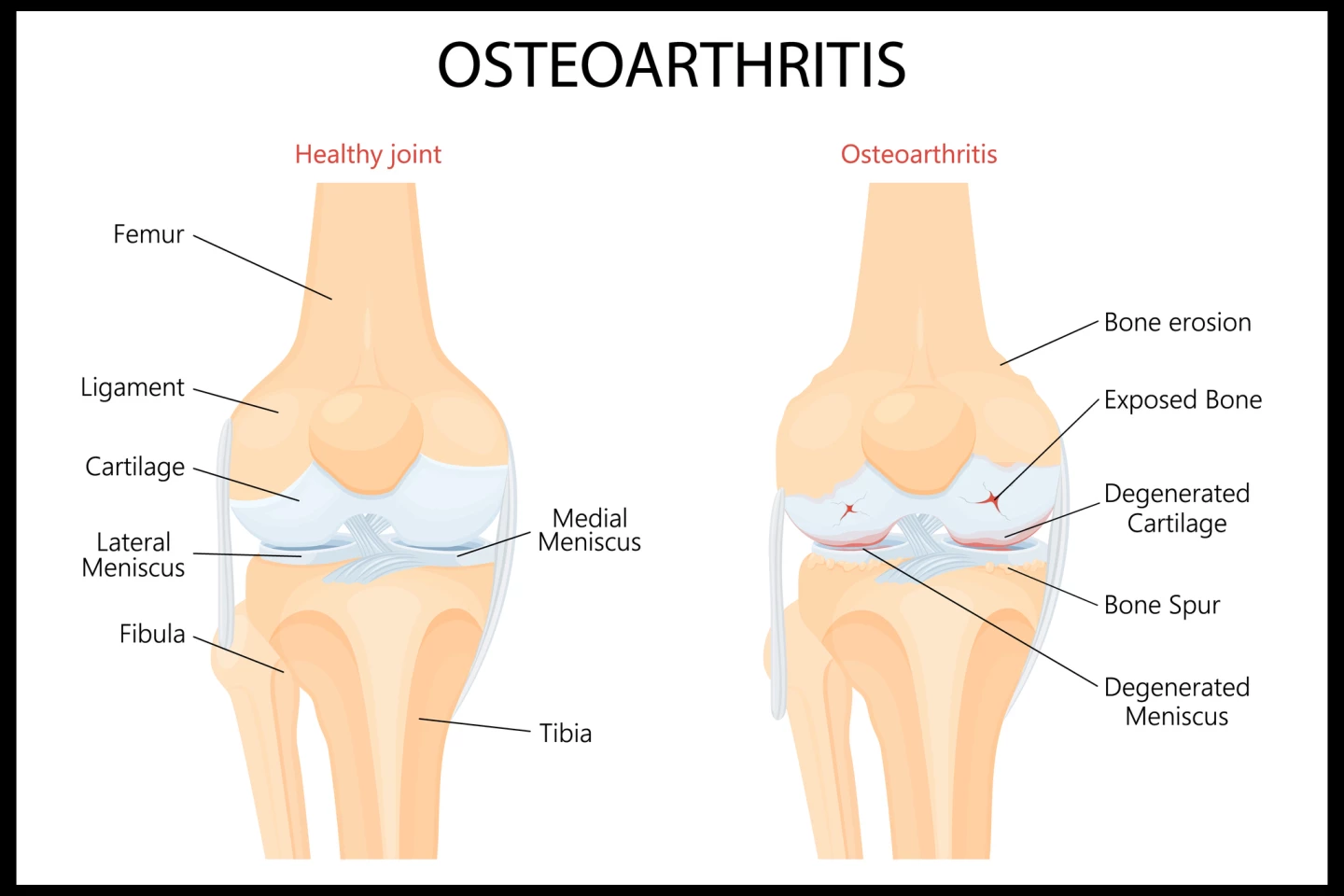
In 2020, 7.6% of the global population had the painful degenerative condition osteoarthritis, an increase of 132.2% in total cases since 1990. It’s expected that by 2050, cases will have increased by 74.9% for knee, 48.6% for hand, and 78.6% for hip osteoarthritis.
Current treatment includes pain relief, usually with non-steroidal anti-inflammatory drugs (NSAIDs), exercise as tolerated, and joint replacement surgery. No available treatment delays the progression of the condition, and long-term use of NSAIDs increases the risk of stomach ulcers, kidney failure, and stroke or heart attack. However, clinical trials of a new one-injection stem cell treatment for osteoarthritis, developed by the private Australian biotech company Magellan Stem Cells, have shown very promising results.
“Our research indicates that Magellan’s off-the-shelf donor stem cell treatment is safe and effective in improving joint function, reducing pain levels, and may have the potential to halt the progression of osteoarthritis,” said Associate Professor Julien Freitag, lead researcher and Magellan’s Executive Director and Chief Medical Officer.
MAG200 is a single injection of donor stem cells into the joint (an intra-articular injection). It’s considered an ‘off-the-shelf’ therapy because it uses donor, or allogeneic, stem cells rather than the patient’s own, the surgical harvesting of which is labor-intensive. Importantly, because the treatment uses mesenchymal stem cells – adult stem cells that can differentiate into other cell types – from adipose tissue or body fat, it doesn’t trigger an immune response.
For the first-in-human Phase I/II trial of MAG200, the researchers randomized 40 participants with moderate knee osteoarthritis to receive either an intra-articular injection of the stem cell therapy or a placebo. All the participants had attempted to manage their osteoarthritis conservatively and had an average pain score of equal to or more than five on a scale of zero to 10. Measures of the impact of their osteoarthritis on function – performing the activities of daily living – were taken using a subscale of the Knee injury and Osteoarthritis Outcome Score (KOOS). KOOS scores go from zero, indicating the worst possible knee symptoms, to 100, indicating no symptoms.
The primary efficacy objective of the study was clinically meaningful differences in pain (a decrease in pain score by two or more points) and function (an eight or more point increase in KOOS score) at 12 months. The researchers found that 75% of participants who’d received MAG200 exhibited clinically relevant and statistically significant improvement in pain and function, reporting improvement or complete recovery. There was sustained pain improvement of 58% at 12 months. MRI scans of the participants’ knees indicated that, at 12 months, those who’d received MAG200 had improvements in cartilage volume and quality.
Given that the goal of osteoarthritis treatment is to avoid surgery to replace diseased joints, the researchers say that their study results are significant.
“More than 30% of total knee replacements in Australia are performed on patients under the age of 65,” Freitag said. “Magellan’s stem cell therapy – with observed pain and functional improvement and indication of disease modification – promises to delay or prevent the later need for joint replacement surgery. I believe our research results represent a pivotal moment for the management of osteoarthritis and may change the way we practice medicine in the future.”
Further research will reinforce the effectiveness of the novel, longer-lasting treatment, which will benefit not only Australians but, eventually, osteoarthritis sufferers worldwide.
“Magellan’s clinical development of its donor cell therapy – MAG200 – provides Australians suffering from osteoarthritis with early access to an innovative therapy which may later have significant impact on the global healthcare and community burden of osteoarthritis,” said Freitag. “If the results of this PI/II trial are replicated by further research, Magellan’s stem cell therapy has the potential to revolutionize the treatment of osteoarthritis, leading to improved quality of life for millions of patients who suffer from this debilitating condition.”
The study was published in the journal Osteoarthritis and Cartilage Open.






