The development of once-a-day pre-exposure prophylaxis (PrEP), drugs like Truvada and Descovy signified a huge advance in HIV medicine. When taken as prescribed by someone who’s HIV-negative, PrEP reduces their risk of contracting the virus from sex by about 99%.
Now, the results of Phase 3 clinical trials undertaken by Gilead Sciences, Inc. have demonstrated that their new injectable PrEP medication, given just twice a year, is 100% effective in preventing HIV spread.
“With zero infections and 100% efficacy, twice-yearly lenacapavir has demonstrated its potential as an important new tool to help prevent HIV infections,” said Merdad Parsey, MD, PhD, Gilead Sciences’ Chief Medical Officer. “We look forward to additional results from the ongoing PURPOSE clinical program and continuing our goal of helping to end the HIV epidemic for everyone, everywhere.”
Lenacapavir is what’s called a capsid inhibitor. In the HIV type 1 (HIV-1) virus, the capsid is a protein shell that houses and protects viral genetic material and is crucial for transporting the virus into a host cell. Once inside the host cell, the capsid is shed, and the virus begins copying itself. Lenacapavir stops that from happening.
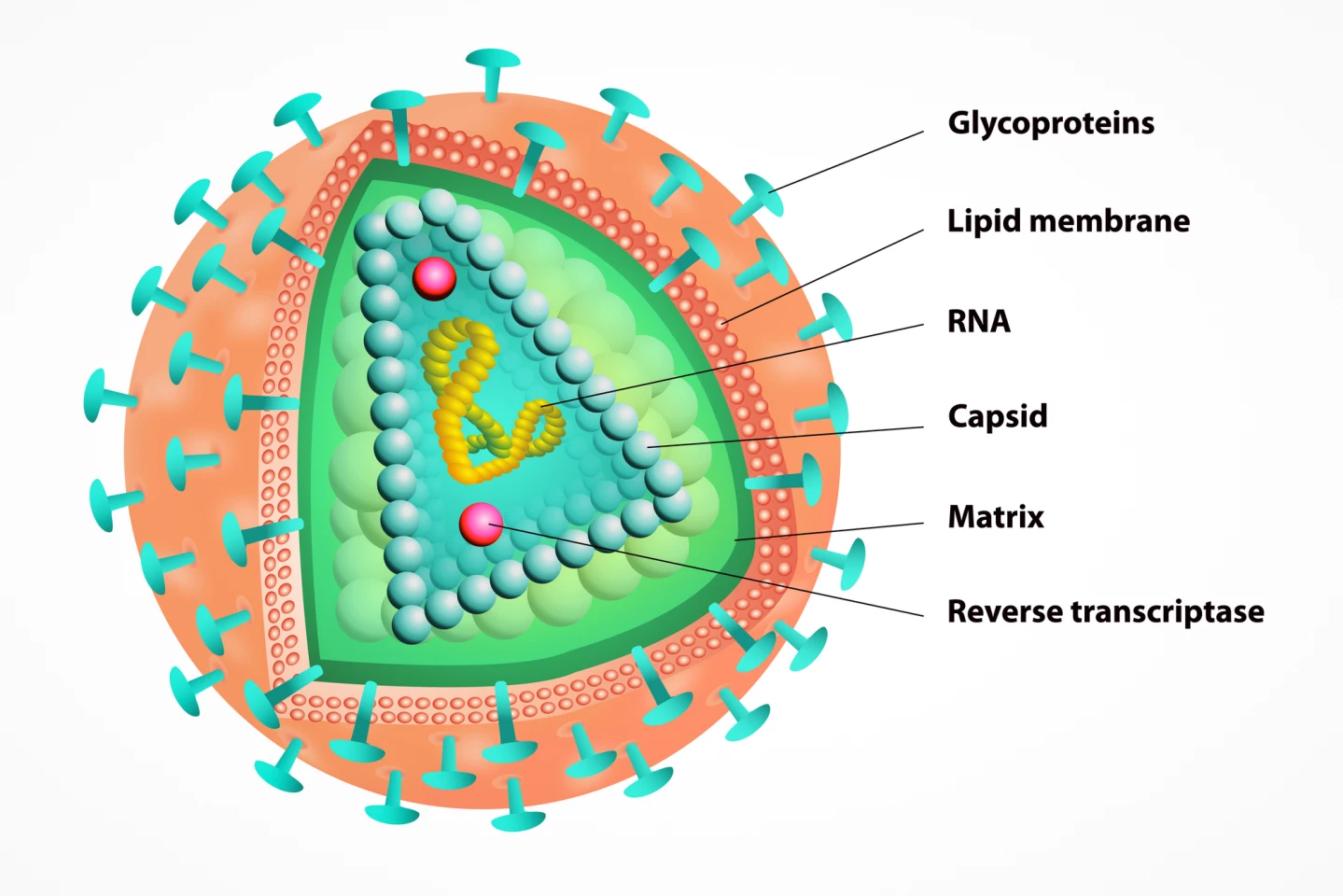
Truvada and Descovy, on the other hand, are nucleoside reverse transcriptase inhibitors (NRTIs) and prevent sexually acquired HIV infection via a different mechanism. They block reverse transcriptase, an enzyme the virus uses to replicate itself. Both drugs contain a combination of antivirals, emtricitabine and tenofovir, but differ in the form of tenofovir they contain. Truvada contains tenofovir disoproxil fumarate (TDF), whereas the newer Descovy contains tenofovir alafenamide (TAF). Gilead Sciences also manufactures Truvada and Descovy.
The first data from Gilead Science’s PURPOSE 1 Phase 3, double-blind, randomized trial, was extremely promising. In it, the safety and efficacy of a twice-yearly, under-the-skin (subcutaneous) injection of lenacapavir for PrEP was compared with once-daily oral Descovy or Truvada in more than 5,300 cisgender women and adolescent girls aged 16 to 25 across 25 sites in South Africa and Uganda. (Cisgender means that a person’s gender identity matches the sex they were assigned at birth.) Because Truvada and Descovy already exist as effective PrEP options, it was considered unethical to include a placebo group.
Zero cases of HIV infection were reported among women in the lenacapavir group, compared with 16 cases in the Truvada group and 39 cases in the Descovy group. Lenacapavir was generally well-tolerated by study participants, and no significant or new safety concerns were observed. Based on these results, the independent Data Monitoring Committee recommended that the blinded phase of the trial be discontinued and that all participants be offered lenacapavir.
Presently, neither lenacapavir nor Descovy are approved for the prevention of HIV in cisgender women. The trial investigators said that their data supports twice-yearly lenacapavir as superior to oral PrEP options, given the challenges of adhering to a daily regimen. This is not to say that existing PrEP drugs are ineffective; rather, they need to be taken as prescribed.
“Twice-yearly lenacapavir for PrEP, if approved, could provide a critical new choice for HIV prevention that fits into the lives of many people who could benefit from PrEP around the world – especially cisgender women,” said Linda-Gail Bekker, Director of the Desmond Tutu HIV Center at the University of Cape Town in South Africa and past President of the International AIDS Society. “While we know traditional HIV prevention options are highly effective when taken as prescribed, twice-yearly lenacapavir for PrEP could help address the stigma and discrimination some people face when taking or storing oral PrEP pills, as well as potentially help increase PrEP adherence and persistence given its twice-yearly dosing schedule.”
The results from another important clinical trial, PURPOSE 2, are expected to be released in late 2024 or early 2025. PURPOSE 2 is assessing the efficacy of twice-yearly lenacapavir for PrEP among cisgender men who have sex with men, transgender men, transgender women and gender non-binary individuals who have sex with people assigned male at birth. The PURPOSE 2 trial is being run in Argentina, Brazil, Mexico, Peru, South Africa, Thailand, and the US.
The PURPOSE trials represent the most comprehensive, diverse HIV prevention trial conducted. Gilead Sciences intends to seek approval for the use of lenacapavir for PrEP using data from both trials to ensure that the drug is accessible to populations and communities in need of additional HIV prevention options.
More detailed data from the PURPOSE 1 trial will be presented at a future conference.
Source: Gilead Sciences







