As marijuana legalization sweeps through the United States there is a pressing need for an accurate roadside test for intoxication. The complexity of the primary psychoactive compound in marijuana is still proving to be a challenge for scientists trying to produce a straightforward breath test. A team at the National Institute of Standards and Technology (NIST) has recently made a major breakthrough in developing a new technique that can detect the presence of marijuana in vapor.
With one recent study by the Highway Loss Data Institute claiming the legalization of marijuana has coincided with an increase in road accidents, some lawmakers are becoming increasingly hesitant about passing new recreational laws. In May the governor of Vermont vetoed a recreational marijuana legalization bill, noting concerns over stoned driving and the lack of an "impairment testing mechanism."
Scientists face several challenges in developing a simple marijuana breathalyzer including the frustrating tendency for THC to linger in the body long after the psychoactive effects of the drug have worn off.
The more pressing fundamental difficulty in producing a functional marijuana breath test has been that the vapor pressure of THC molecules prove to be notoriously difficult to identify. Ethyl alcohol molecules have a high vapor pressure, meaning many molecules escape as gas when in liquid form. This is why it is easy to identify blood alcohol levels through the breath of an intoxicated person.
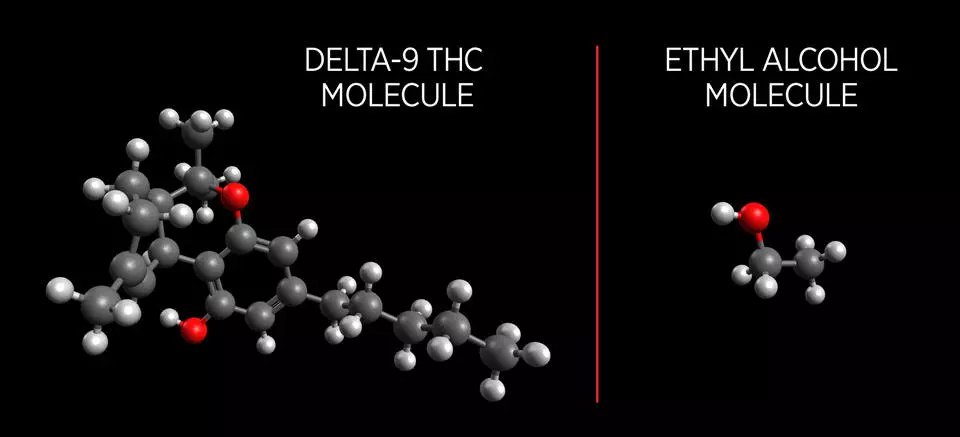
THC molecules on the other hand are larger and more complex with a low vapor pressure. This makes developing a measurement device that can identify THC in a person's breath incredibly difficult. The team at NIST tackled this problem by using a new process called PLOT-cryo.
"PLOT-cryo is an extremely sensitive technique for capturing and analyzing things in the vapor phase," say co-author of the study, Tom Bruno. "It was a natural candidate for this type of problem."
Invented in 2009, the PLOT-cryo system has been used to detect trace elements of explosives in airports and find buried bodies by identifying faint scents of decomposition in the air. In this study the NIST team successfully used the technique to calculate the vapor pressure of both THC and cannabidiol, a second, less psychoactive compound found in marijuana.
Despite this foundational technique, more research is still needed before we will see a reliable marijuana breathalyzer. An accurate understanding of how THC vapor pressure levels correlate with blood levels is yet to be researched. There also isn't yet an agreed scientific consensus over what level of THC in the blood constitutes a safe driving volume.
The NIST research certainly offers a strong scientific basis in a technique that can accurately measure THC levels in vapor, hopefully spurring further research that can produce an accurate and reliable marijuana breathalyzer in the future.
Source: NIST




