By fitting microparticle 'backpacks' to important inflammatory cells called macrophages, researchers significantly reduced lesion size and inflammation caused by traumatic brain injury. Working with biology rather than against it, this novel approach has the potential to be an effective treatment for a debilitating condition.
Globally, in 2019, there were 27.16 million new cases of traumatic brain injury (TBI) and 48.99 million cases of people with existing TBI. While neuroinflammation is important for promoting cell and tissue regeneration immediately following a TBI, prolonged inflammation can lead to secondary injury. The activation of brain-resident macrophages – white blood cells that can switch between pro- and anti-inflammatory states – and other immune cells can expand the lesion and increase the likelihood of complications like depression, sensorimotor and memory deficits, and dementia.
Now, researchers from the Wyss Institute at Harvard University have recruited macrophages to aid in treating TBI. Fitted with a microparticle “backpack” filled with anti-inflammatory molecules, the kitted-out macrophages significantly reduced local brain inflammation, lesion size and bleeding in pigs with TBI.
“Every year, millions of people suffer from a TBI, but there is currently no treatment beyond managing symptoms,” said Samir Mitragotri, one of the study’s corresponding authors. “We have applied our cellular backpack technology – which we previously used to improve macrophages’ inflammatory response to cancerous tumors – to deliver localized anti-inflammatory treatment in the brain, which helps mitigate the cascade of runaway inflammation that causes tissue damage and death in a human-relevant model.”
As brain cells die due to a traumatic impact, they release a cocktail of pro-inflammatory cytokines that attract immune cells to repair the damage. But these cytokines can also disrupt the blood-brain barrier, causing blood to leak into the brain, leading to swelling, impaired oxygen delivery, and increased inflammation. It’s a vicious cycle of bleeding and damage that drives even more cell death.
Based on their previous work fitting backpacks to macrophages, the researchers figured they could be effective in cases of TBI.
“It’s generally believed anti-inflammatory therapies can be effective for treating TBI, but, so far, none of them have proven effective clinically,” said Rick Liao, the study’s co-lead author. “Our previous work with macrophages has shown us that we can use our backpack technology to effectively steer their behavior when they arrive at the injury site. Since these cells are already active players in the body’s natural immune response to a TBI, we had a hunch we could augment that preexisting biology to reduce the initial damage.”

The cellular backpacks are comprised of two outer layers of dexamethasone, an anti-inflammatory steroid medication, in poly (lactic-co-glycolic acid) (PLGA) and a middle layer of IL-4, an immunoregulatory cytokine, in poly(vinyl alcohol) (PVA). Combining dexamethasone and IL-4 has been shown to induce a synergistic anti-inflammatory effect. The backpacks have an average diameter of 8.2 µm and a thickness of 914 nm and are designed to adhere to the surface of macrophages.
Pig macrophages were cultured from bone marrow and backpacks attached to create a backpack-macrophage complex. The complexes were administered intravenously to pig models of TBI with an injury to the cortex, the outermost layer of brain tissue. Seven days later, the backpack-macrophages were observed in the injury lesion in greater density than in other brain areas.
Compared to pigs that received a saline injection, treatment with the backpack-macrophages reduced total macroscopic lesion volume by 56% and produced a “visually striking” reduction in the amount of hemorrhaging (73 mm3 versus 21 mm3). Hemorrhage volume was positively correlated to lesion volume, indicating that it was heavily impacted by how much the lesion hemorrhaged as it evolved. Backpack-macrophages reduced microscopic lesion volume, an assessment of permanent tissue damage, by 47%. Pigs treated with the backpacks were also seen to have fewer large lesions.
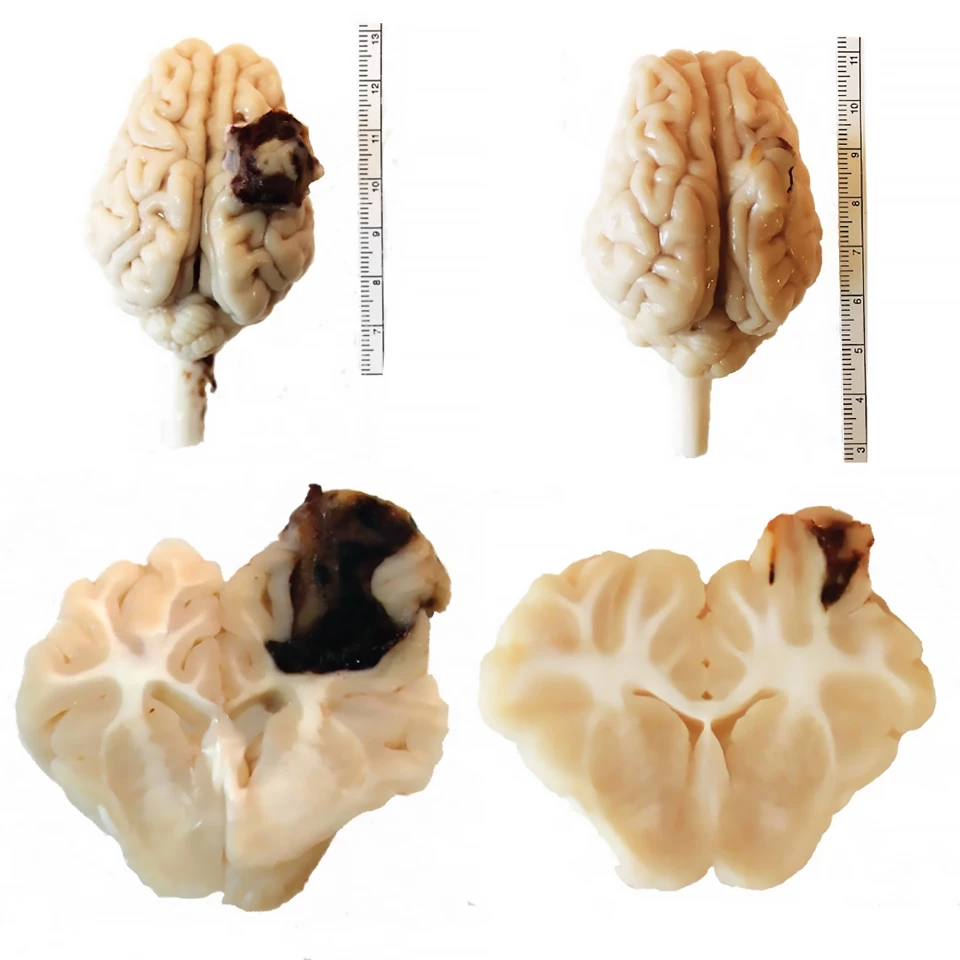
The researchers analyzed the microglia, the brain’s injury-repairing cells, in the lesions of treated pigs and found a reduction in the pro-inflammatory marker CD80 compared to the saline group. Peripheral biomarkers of inflammation were also less in backpack-macrophage-treated pigs. Twenty-four hours after injury, serum tumor necrosis factor alpha (TNF-⍺), a major regulator of inflammatory responses, was 82.7% in treated pigs compared to 117.5% in controls. Seven days post-injury, serum glial fibrillary acidic protein (GFAP), a diagnostic and prognostic biomarker of TBI and neuroinflammation, was less in treated (75.2%) versus saline (158.4%) groups.
The rate of adverse events for treated pigs did not differ from that of the animals that received saline. No signs of toxicity due to backpack-macrophage treatment were observed in the spleen, liver, kidney, and lungs.
“Macrophages’ susceptibility to their local environment has historically prevented scientists from taking full advantage of their immune-modulating capabilities,” said the Wyss Institute’s Founding Director, Donald Ingber, who was not involved in the research. “This impressive study describes a truly novel and potentially powerful macrophage-based therapy for treating inflammation that is the root cause of so many human afflictions in an effective and non-invasive way that works with biology rather than against it.”
The study was published in the journal PNAS Nexus.
Source: Wyss Institute at Harvard







