A new study has demonstrated that a natural bacterial defense mechanism against invading viruses can be used as a weapon to combat bacterial infection. The finding opens the door to new anti-bacterial therapeutics, particularly important in the face of rising antibiotic resistance.
When certain bacteria are infected by a virus, they initiate a self-destruct mechanism called the cyclic oligonucleotide-based antiphage signaling system (mercifully shortened to CBASS) to prevent the spread of that virus to other bacterial cells in the population. Researchers at the Icahn School of Medicine at Mount Sinai have examined this natural bacteria-killing mechanism more closely to see how it works and whether it could be harnessed to control bacterial infections.
“We wanted to see how the bacterial self-killing CBASS system is activated and whether it can be leveraged to limit bacterial infections,” said Aneel Aggarwal, the study’s co-corresponding author. “This is a fresh approach to tackling bacterial infections, a significant concern in hospitals and other settings. It’s essential to find new tools for fighting antibiotic resistance. In the war against superbugs, we need to constantly innovate and expand our toolkit to stay ahead of evolving drug resistance.”
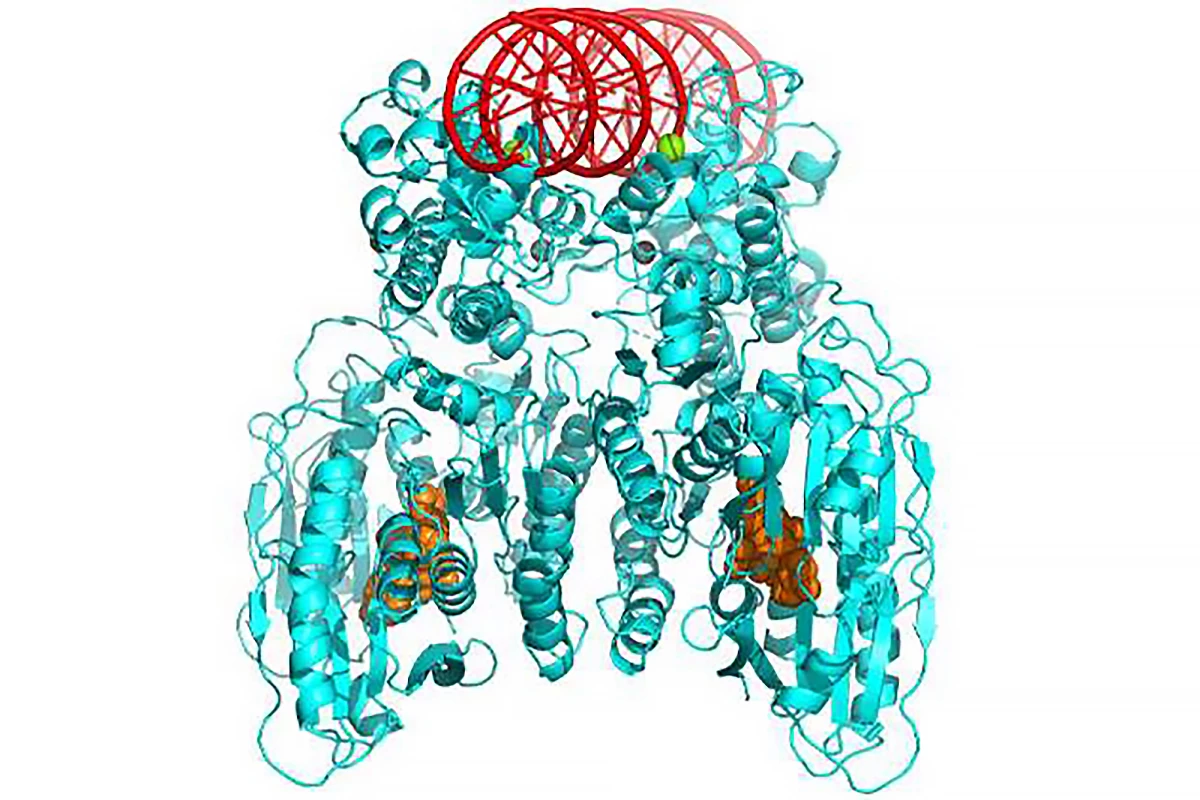
The researchers looked particularly at CBASS-associated protein 5 (Cap5) in Pseudomonas bacteria. Cap5 is activated by cyclic nucleotides, small signaling molecules or ‘second messengers’ that are synthesized when a viral infection is sensed and bind to an effector protein to degrade the bacteria’s DNA, causing its death. They were able to obtain high-resolution structures of Cap5 from Pseudomonas in a complex with cyclic nucleotides, uncovering the key to their activation.
“In our study, we started by identifying which of the many cyclic nucleotides could activate the effector Cap5 of the CBASS system,” said Olga Rechkoblit, the other corresponding author. “Once we figured that out, we looked closely at the structure of Cap5 when it’s bound to these small signaling molecules. Then … we showed that by adding these special molecules to the bacteria’s environment, these molecules could potentially be used to eliminate the bacteria.”
The researchers say their study provides proof-in-principle evidence that the self-destructive activity of CBASS effector proteins like Cap5 can be harnessed to limit bacterial infection, which may lead to new anti-bacterial therapeutics. Further research will explore how their findings apply to other types of bacteria and whether their method can be used to manage harmful bacterial infections.
The study was published in the journal Nature Structural & Molecular Biology.
Source: Mount Sinai