Stanford researchers are claiming to have optimized an already FDA-approved form of non-invasive magnetic brain stimulation to better treat those suffering from severe, treatment-resistant depression. A small preliminary study achieved an extraordinary 90 percent remission rate, and larger trials are now underway.
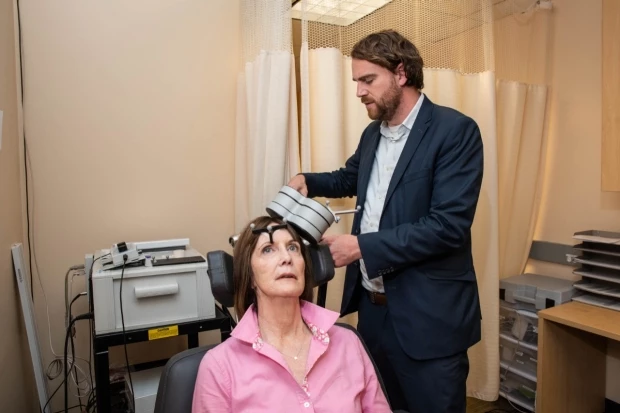
In 2008 the US Food and Drug Administration (FDA) approved the first magnetic brain stimulation device to treat major depressive disorder. Called repetitive transcranial magnetic stimulation (rTMS), the treatment involves applying noninvasive magnetic pulses to specific regions of the brain.
Generally, rTMS has only been mildly better than placebo at improving depressive symptoms in trials, so its clinical uses have mostly been limited to severe cases of treatment-resistant depression, when every other option has failed. The new Stanford study is testing a novel form of the treatment, called Stanford Accelerated Intelligent Neuromodulation Therapy, or SAINT.
“There’s never been a therapy for treatment-resistant depression that’s broken 55-percent remission rates in open-label testing,” says senior author on the new study, Nolan Williams. “Electroconvulsive therapy is thought to be the gold standard, but it has only an average 48-percent remission rate in treatment-resistant depression. No one expected these kinds of results.”
The new SAINT technique alters the traditional rTMS treatment in a few key ways. Prior rTMS treatments generate around 600 magnetic pulses per session, whereas the SAINT technique triples that dose to 1,800 pulses per session.
The treatment schedule was also significantly tweaked by the Stanford team. Instead of the usual one session per day for six weeks schedule, the new method is more aggressive, administering 10 sessions a day, lasting 10 minutes per session. Most subjects tested reported positive results after just three days of this kind of regime.
The other improvement suggested by the Stanford research is more accurate targeting of the magnetic pulses. To treat depression, the rTMS technique generally targets the dorsolateral prefrontal cortex. The new SAINT technique aims the stimulation at a very specific subregion of the dorsolateral prefrontal cortex, close to the subgenual cingulate. Each patient is individually scanned using magnetic-resonance imaging to personalize the targeting to each unique brain.
The preliminary study tested the SAINT technique on 21 subjects, all of which were suffering from severe depression with suicidal thoughts. All 21 subjects had tried, and not benefited from, antidepressant medications, electroconvulsive therapy, and the current FDA-approved rTMS technique.
After up to five days of the SAINT treatment, 19 of the subjects did not register as clinically depressed according to several traditional diagnostic tests. All 21 subjects reported no suicidal thoughts after the treatment, and one month after the treatment 60 percent of the subjects were still in remission.
Deirdre Lehman, one of the subjects in the study, says after just one day of the SAINT treatment a constant chattering in her brain had subsided.
“By the third round, the chatter started to ease,” says Lehman. “By lunch, I could look my husband in the eye. With each session, the chatter got less and less until it was completely quiet. That was the most peace there’s been in my brain since I was 16 and started down the path to bipolar disorder.”
Of course, it is important to note this is a small preliminary study with no placebo control or blinding. A larger, double-blind trial is already underway, so hopefully there will soon be better data to understand how efficacious this new kind of rTMS treatment is. Nevertheless, these results are incredibly promising, and considering rTMS therapies are already approved and being widely administered, this tweaking of the treatment could result in an easily deployable improvement helping patients with severe treatment-resistant depression.
The new study was published in the American Journal of Psychiatry.
Source: Stanford Medicine




