Researchers have identified a blood protein that can be used as a marker of gastric and other cancers and is more accurate than existing diagnostic biomarkers, even in the early stages of the disease. The simple test may lead to the earlier diagnosis of often stealthy gastrointestinal cancers.
Gastrointestinal cancers, which include esophageal, gastric, colorectal, liver, and pancreatic cancers, often go undetected. The late discovery of these cancers can impact the effectiveness of treatment and contributes to mortality rates.
While there are biomarkers used to detect cancer, they can be inaccurate and don’t always pick up all types, or they are technically complex and expensive to measure. Researchers from Nagoya University have identified a protein that is a reliable marker of gastric cancer, even in the early stages of the disease, and can be tested using a simple blood test.
“Currently, blood tests to detect cancers, such as gastric, colorectal, and breast cancer, have used tumor markers like CEA and CA19-9,” said Takahiro Shinozuka, the study’s lead author. “However, these tumor markers do not always accurately detect all cancers, and their accuracy needs to be improved. Other markers have been proposed but have drawbacks, such as intricate, costly measurement procedures or invasive testing methods, that prevent their use.”
Carcinoembryonic antigen (CEA) is produced by colorectal cancer cells and released into the blood. However, not all colorectal cancers produce CEA, so a normal level doesn’t necessarily rule out cancer. Levels can also be elevated in breast, lung, and thyroid cancers, and CEA can be produced in non-cancerous conditions like liver disease, pancreatitis and ulcerative colitis. Meanwhile, carbohydrate antigen 19-9 (CA19-9) is released by pancreatic, gallbladder, lung and colon cancer cells, but levels can also be elevated in diabetes, pancreatitis, and other lung, urological and gynecological diseases.
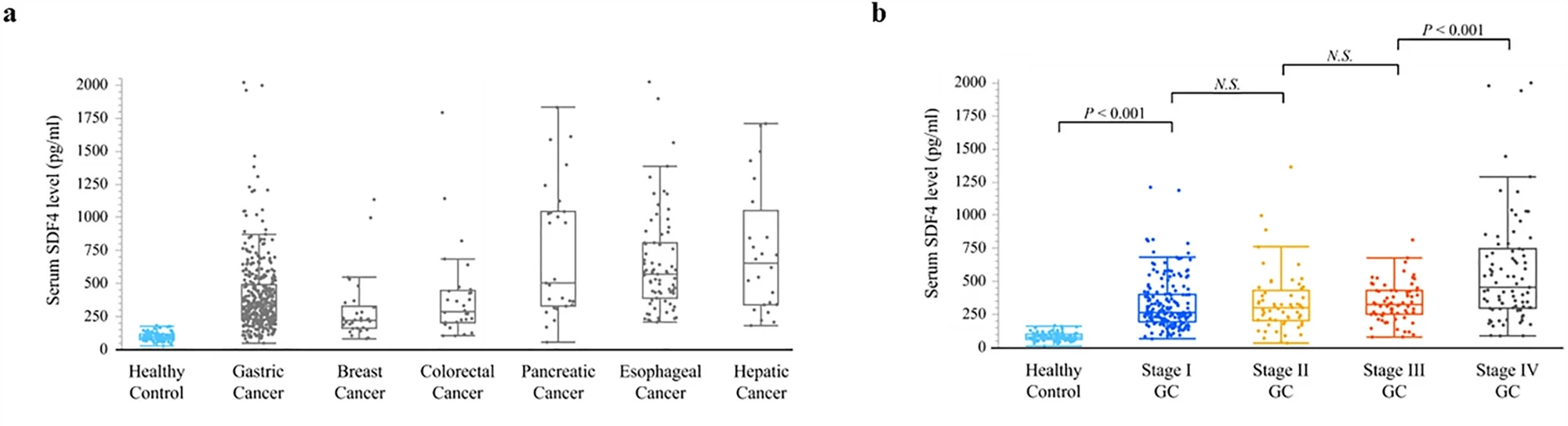
The researchers used a dataset of previously reported secretomes – proteins expressed by cells, tissues and organs – using gastric cancer cell lines to identify a new diagnostic biomarker that could be detected in blood. They found that stromal cell-derived factor 4 (SDF4) was a potential serum diagnostic tumor marker.
They collected blood samples from 582 patients with gastric, breast, colorectal, pancreatic, esophageal and liver cancer, and 80 healthy controls, and found that SDF4 was consistently elevated in the cancer samples. In gastric cancer patients, the SDF4 level increased with the clinical stage, and levels were significantly higher in patients with stage 1 gastric cancer compared with healthy controls, suggesting that the test could detect gastric cancer early, before symptoms appeared.
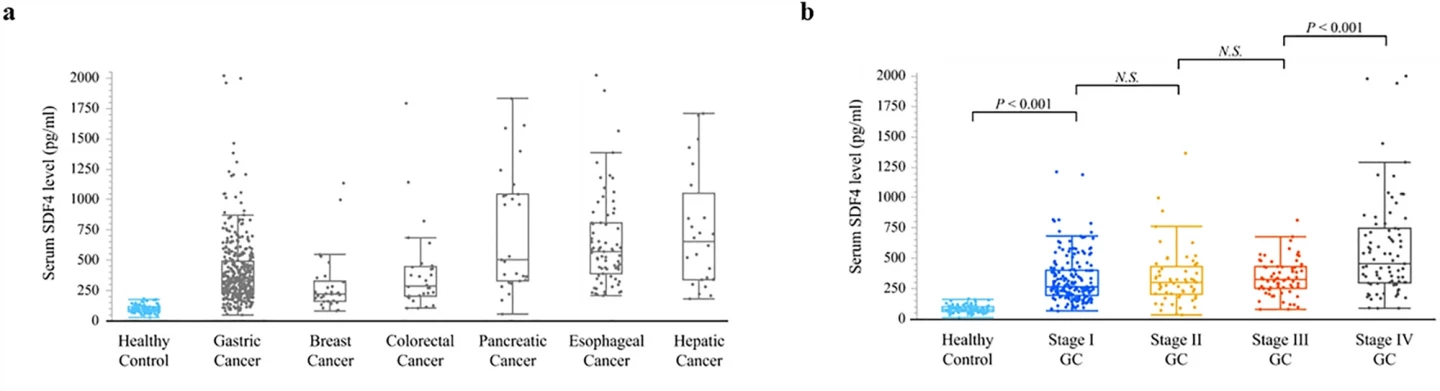
The researchers found that, as a biomarker of gastric cancer, SDF4 had a sensitivity of 89% and a specificity of 99%. Sensitivity shows how well the test finds the disease in sick patients, whereas a test’s specificity is its ability to designate an individual who does not have the disease. A highly sensitive test means that there are few false negative results. SDF4 outperformed existing cancer biomarkers in terms of sensitivity: 13% for CEA and 17% for CA19-9.
The researchers recognize that a major limitation of the study was that the sample size was small and came from a single Japanese institution that may not be representative of other patient populations. Future studies using a larger cohort are necessary to determine the diagnostic significance of SDF4 and translate the findings to clinical practice. However, they see potential in using SDF4 as a diagnostic marker of gastric and other cancers.
“There are two ways in which SDF4 outperforms conventional tumor markers as a diagnostic marker,” Shinozuka said. “The first is that it can diagnose patients with early-stage cancer, and the second is that it is useful as a diagnostic marker for various types of cancer. We are working with a company to develop measurement devices that can be used for cancer screening. If these efforts are successful, we hope to introduce SDF4 into actual cancer screening, helping in the early detection of cancer.”
The study was published in the journal Scientific Reports.
Source: Nagoya University