With an industry expected to be worth US$100 billion by 2030, an ongoing supply crisis, a time-consuming manufacturing process, a high price tag and no generic alternatives, it's no surprise the much-hyped class of injectable weight-loss drugs has become a prime target for counterfeit trade.
Now, international authorities and drug companies have issued new warnings in relation to bogus medicines, after several people in Austria were admitted to hospital following use of fake Ozempic pens they reportedly obtained from a doctor. The patients suffered serious health issues including hypoglycemic shock and coma, leading the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) to believe the counterfeit pens contained insulin not semaglutide – Ozempic’s active ingredient.
"We are advising all members of the public not to use any pre-filled weight-loss pens they may have bought online and instead to report it to us so that we can investigate and take any necessary action," said the MHRA in a statement.
Since January 2023, the MHRA has seized 369 Ozempic pens that are thought to be fake. There have been no reports of counterfeits of the drug prior to this year, with authorities predicting a rise in illegal trade.
“No-one should put profit before the needs of patients, but fraudsters selling black market medicines like this are extremely dangerous and can put people’s health at risk,” said UK Health Minister Will Quince. “The medical advice is clear: patients should only use medicines like Ozempic or Saxenda where they’ve been prescribed it by a legitimate source, such as their GP or another legitimate prescriber.
“The MHRA have our full support in cracking down on these illegal online suppliers to ensure that patients are protected.”
Semaglutide belongs to the class of medicines known as glucagon-like peptide-1 (GLP-1) receptor agonists, used for diabetes management but increasingly for weight loss. The two brands of the drug currently on the market – Ozempic and Wegovy – are made by Denmark’s Novo Nordisk and have been in short supply globally for much of 2023. Novo Nordisk also makes the similar GLP-1 drug liraglutide, which is sold for weight-loss treatment in the UK as Saxenda.
A third drug, tirzepatide (sold as Mounjaro from US company Eli Lilly and Company), has been on the market since 2022 for type 2 diabetes treatment, however, its recent approval for weight-loss prescription has also seen it in drastically short supply. It activates the same hormone receptor as Ozempic, as well as another related one. (Triple agonists, which activate three receptors, are in development.)
Compounding supply issues is the manufacturing processes for the drugs. Ozempic requires specialized equipment and a unique setting, so scalability beyond current measures is not simple.
“Buying products such as Ozempic or Saxenda without a prescription, from illegally trading suppliers, significantly increases the risk of receiving something which is either fake or not licensed for use,” said Dr Alison Cave, MHRA’s Chief Safety Officer.
Germany’s Federal Institute for Drugs and Medical Devices (BfArM ) has also ramped up its investigations and issued new warnings, after 1-mg Ozempic pens inside German packaging were identified at a wholesale level.
“So far there is no evidence that the counterfeits have reached patients in Germany,” BfArM said in a statement identifying the serial numbers of the fake products. “However, it cannot be ruled out that counterfeit medicines are in the legal distribution chain in Germany.
“The outer packaging of the medicine is difficult or impossible to distinguish from the original,” the statement continued. “However, the counterfeit can be easily identified visually on the primary packaging.”
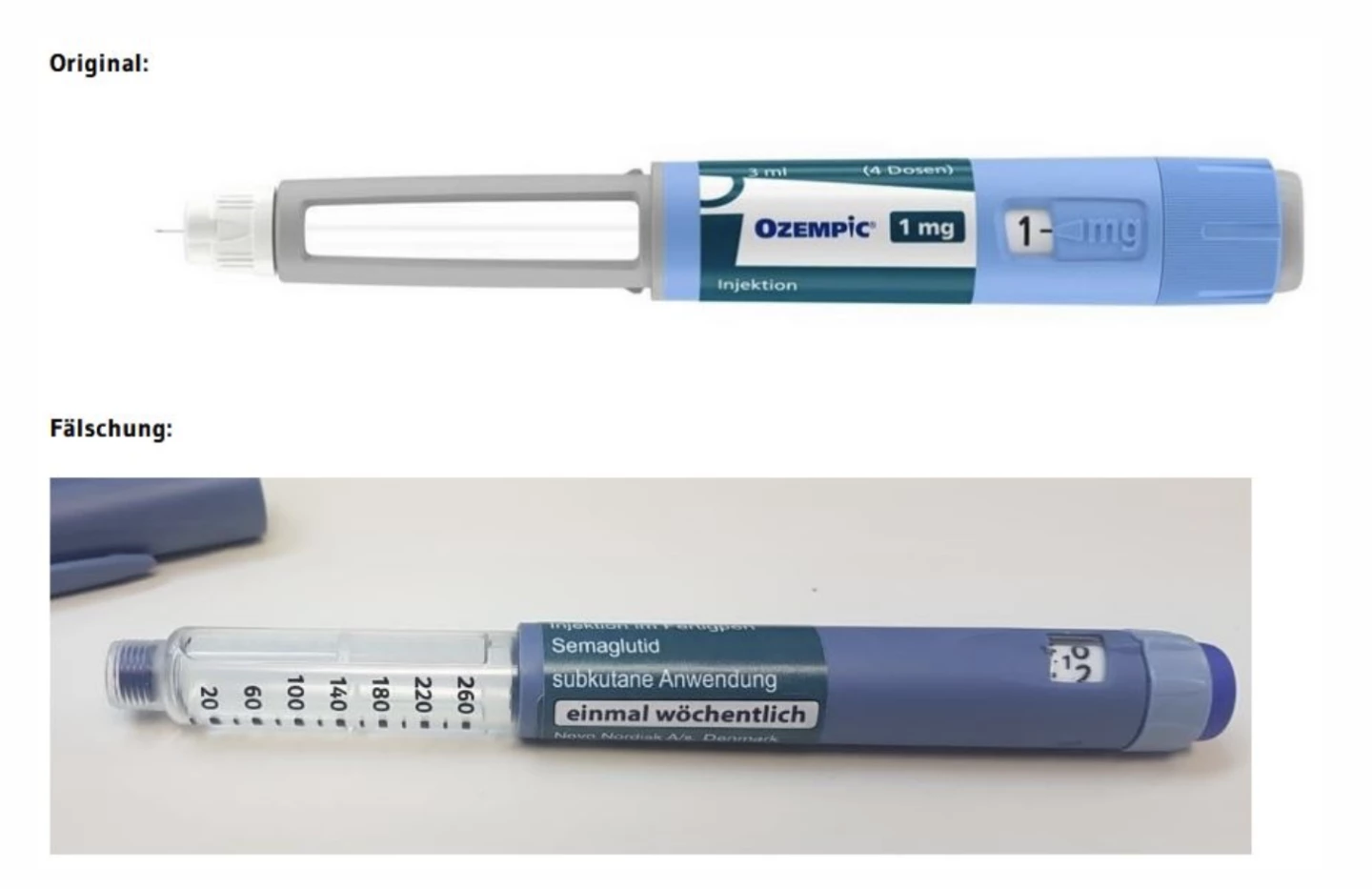
As such, pharmacies must open and inspect all boxes before dispensing to the patients with prescriptions.
In the US, the drug shortages and prohibitively high prices for many consumers has led to a rise in the prevalence of what's said to be compounded semaglutide being sold through compounding pharmacies.
Compounded drugs mix, combine or alter ingredients to tailor medications to individual patient needs, such as removing an allergen from an FDA-approved medication or altering its form (liquid instead of tablet). Around 1-3% of all prescription medications are compounded.
When in short demand, compounding can keep the supply chain of certain medications running, however, semaglutide is not approved for altering in this way.
“Patients should be aware that some products sold as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products and may be the salt formulations,” the FDA stated in June. “Products containing these salts, such as semaglutide sodium and semaglutide acetate, have not been shown to be safe and effective.
“Purchasing medicine online from unregulated, unlicensed sources can expose patients to potentially unsafe products that have not undergone appropriate evaluation or approval, or do not meet quality standards,” said the FDA. “If you choose to use an online pharmacy, the FDA’s BeSafeRx campaign resources and tools can assist in making safer, more informed decisions when purchasing prescription medicine online.”
In June and July, Novo Nordisk launched a series of lawsuits targeting compounding pharmacies and ‘wellness’ centers for using its products' trademarks on non-FDA-approved medications.
"Our priority is to ensure that patients have a safe and positive experience with our FDA-approved semaglutide medicines, and these actions are a direct reflection of that focus," said Doug Langa, executive vice president, North America operations and president of Novo Nordisk. "We believe it's important to provide additional tools and education to support the proper use of our approved semaglutide products and create broad public awareness regarding the difference between our FDA-approved medicines and other products being labeled as semaglutide."
Meanwhile, Mounjaro manufacturer Lilly followed suit, quite literally, in September, taking action to “protect patient safety and stop the unlawful marketing and sale of non-FDA approved compounded products fraudulently claiming to be Mounjaro (tirzepatide) by medical spas, wellness centers and compounding pharmacies.”
All cases are still pending.
More than nine million prescriptions for Ozempic were written in the final quarter of 2022. Novo Nordisk is now the biggest money-maker in Denmark, and the company expects to round out the year with a 40-46% growth in profits when the latest financial figures are released soon.
Source: MHRA






