Scientists at the Scripps Research Institute have spent the past few years studying a promising but problematic signaling pathway they believe can be targeted to prevent type 2 diabetes. The team has now demonstrated how this pathway can be temporarily activated to improve metabolic health in obese mice, blocking the deleterious effects of a high-fat diet.
Around 30 million people are afflicted by type 2 diabetes in the US, with the condition driven primarily by poor diet and inactive lifestyles. This causes persistently high blood sugar, which over time compromises the production of beta cells in the pancreas and, in turn, the ability of insulin to help the body process glucose. Once it takes hold, type 2 diabetes increases the risk of heart disease and stroke, along with other health issues.
The team at the Scripps Research Institute has been searching for new ways to intervene in this dangerous chain of events, and has been investigating a signaling pathway that involves a pair of proteins called IRE1 and XBP1s. Cellular stress driven by obesity can cause IRE1 to activate XBP1s, which then activates a set of genes – many of them metabolic genes – as a protective response.
Previous research had shown that these obesity-related cellular stresses can promote the development of diabetes, and that activity of this pathway can protect liver and fat cells in the short term. But targeting the pathway with drugs hasn't been so straightforward, as constant activation of it leads to inflammation and damage to the cells, ultimately leading to even worse outcomes for metabolic health.
"IRE1/XBP1s signaling is a response to cellular stress, and keeping it on all the time essentially tells the cell that the stress can't be resolved – so the cell in effect kills itself," said Luke Wiseman, who led the research.
The scientists have now found success using an experimental compound they've been working with for a few years. Called IXA4, the scientists found they could use to it activate the pathway but only for a few hours, leaving it switched off the rest of the time.
This was demonstrated in obese mice being fed a high-fat, high-calorie diet, and the team found after eight weeks of treatment the rodents exhibited improved glucose metabolism and insulin activity compared to a control group. They also had less fat buildup and inflammation in the liver, and no loss of insulin-producing cells in the pancreas.
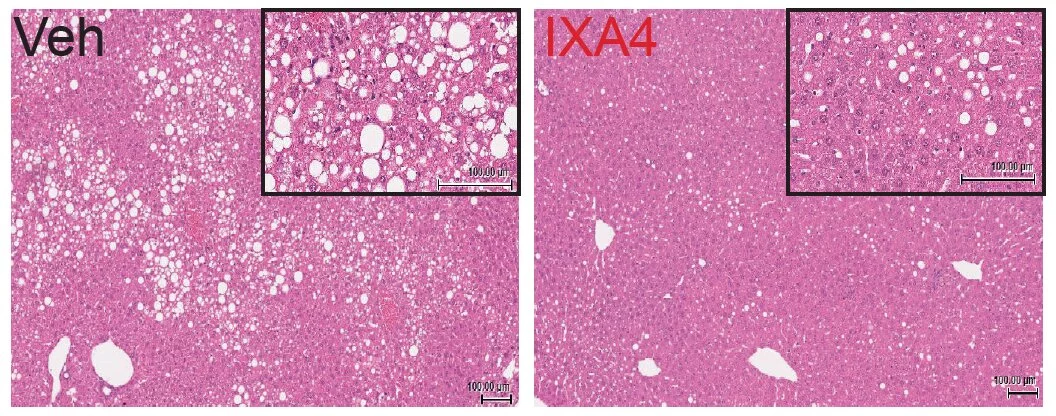
"This is the first time anyone has shown that a small molecule activating this pathway in this manner works to treat disease in a live animal," said Enrique Saez, study author.
Though the treatment led to a "significant overall improvement in metabolic health," the scientists note it can only reach a limited number of tissues, including the liver and the pancreas. They are now working to develop other compounds that can confer similar effects on a wider variety of cells, in order to treat a wider variety of conditions.
"We're also continuing to work with IXA4 as a potential treatment for other metabolic disorders such as fatty liver disease," Saez said.
The research was published in the journal Nature Communications.
Source: Scripps Research Institute via MedicalXpress




