More than one in three American adults has prediabetes, where their blood glucose levels (BGLs) are higher than normal but not high enough to attract a diagnosis of type 2 diabetes. Not everyone with prediabetes goes on to develop diabetes; early treatment can prevent or delay that progression.
A new prediabetes drug called APHD-012, developed by biopharmaceutical company Aphaia Pharma, has undergone a Phase 2 clinical trial to evaluate its performance and produced very promising results.
“We are excited to demonstrate for the first time that the mechanism of action of APHD-012 translates into clinical efficacy,” said Steffen-Sebastian Bolz, Aphaia Pharma’s chief scientific officer. “In addition to showcasing the potential of our oral glucose formulation to manage prediabetes progression, it also underscores its promise to deliver clinical benefits across various indications (e.g., diabetes, obesity, among others), given its mechanism of action in restoring natural hormone release patterns in the intestine.”
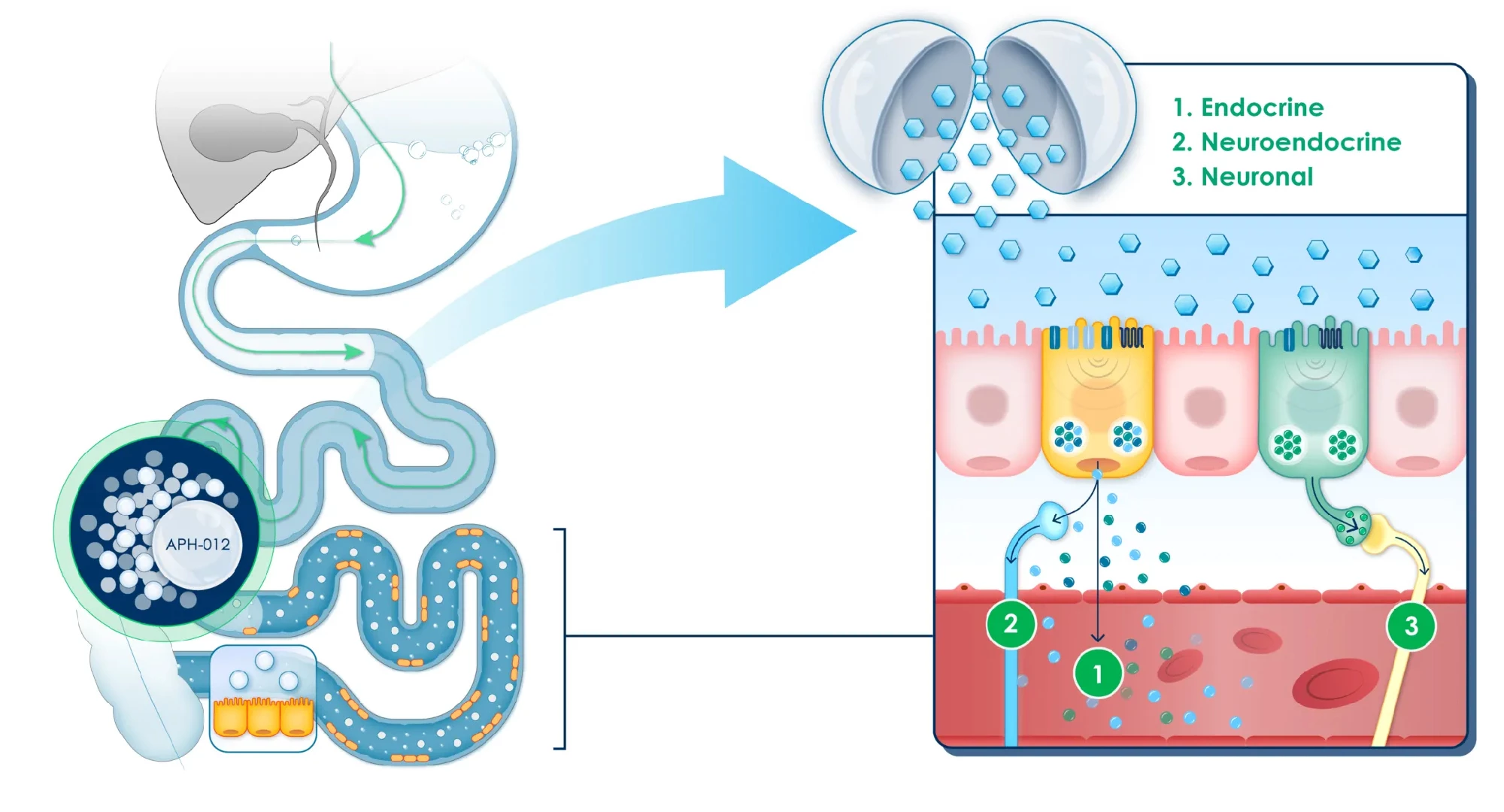
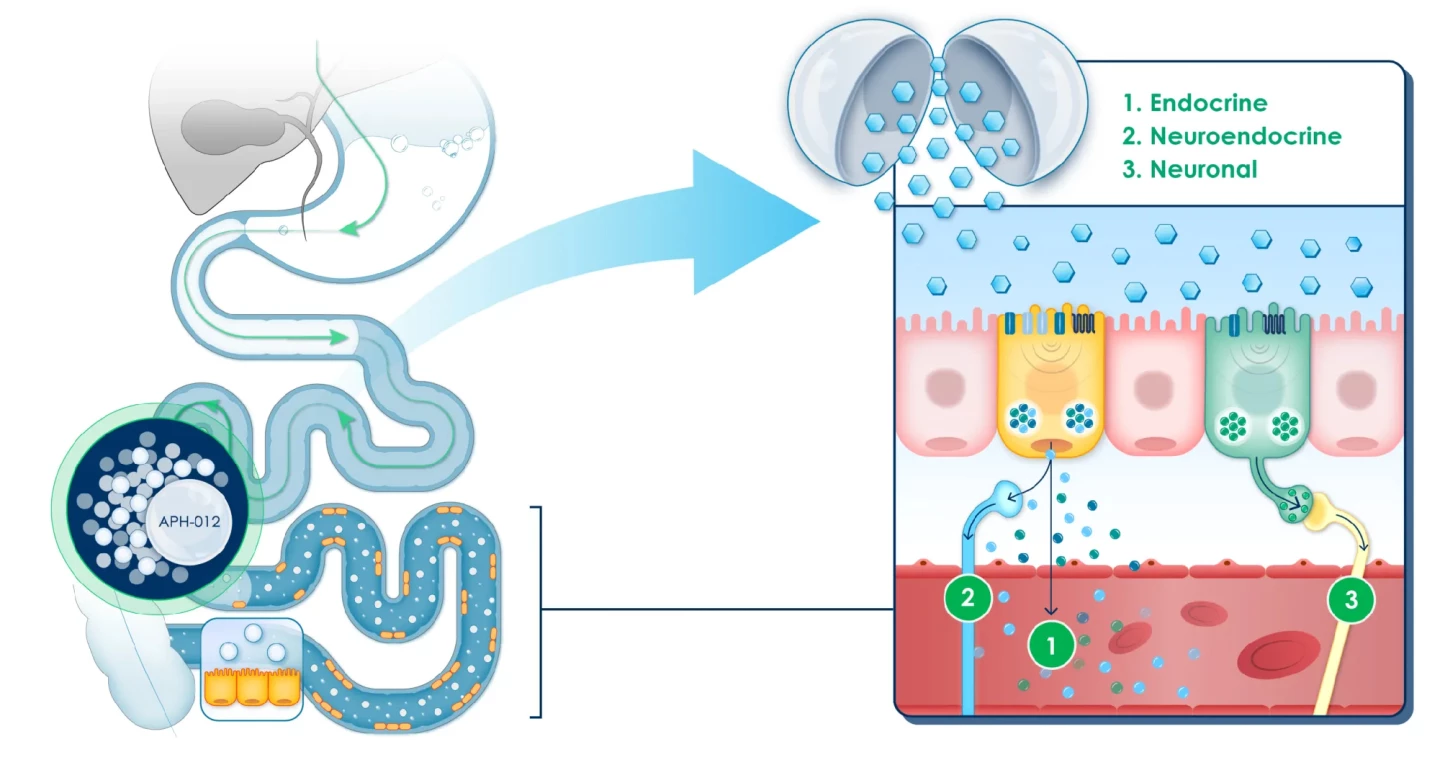
In healthy people, food nutrients are absorbed in a balanced way along the length of the small intestine. Those not absorbed by the upper small intestine, closest to the stomach, travel to the lower small intestine. There, they stimulate nutrient-sensing cells, triggering the release of hormones called incretins that regulate functions like appetite and metabolic activity, including insulin secretion by the pancreas.

In prediabetics, almost all nutrient absorption occurs in the upper small intestine, reducing the hormonal response that would normally occur downstream. APHD-012, an oral formulation of coated glucose beads, is designed to release glucose in pre-determined parts of the lower small intestine to restore and maximize the cells’ nutrient-sensing response and activate the important hormone signaling pathway.
For the randomized clinical trial, about 30 prediabetic, diabetic, and healthy participants received a once-daily dose of APHD-012 or a placebo for six weeks. The primary endpoint was improved glucose tolerance, measured by an oral glucose tolerance test (OGTT).
An OGTT shows how the body handles the ingestion of sugar and is used as a screening test for prediabetes and type 2 diabetes. An individual who’s been fasting for at least eight hours drinks a syrupy glucose solution – usually containing 75 g of sugar – and their BGL is checked two hours afterwards. Someone with prediabetes or diabetes will have an elevated blood sugar post-OGTT. The normal fasting BGL range is 3.9 mmol/L to 5.6 mmol/L (70 mg/dL to 100 mg/dL).
At two hours post-OGTT, APHD-012 reduced BGLs across all trial subjects from 9.0 mmol/L to 7.9 mmol/L (162 mg/dL to 142 mg/dL), a statistically significant improvement. The effect was greater in prediabetic subjects, whose BGLs fell from 8.7 mmol/L to 6.8 mmol/L (157 mg/dL to 122 mg/dL). In the diabetic subjects, APHD-012 produced a drop from 13.2 mmol/L to 11.6 mmol/L (238 mg/dL to 209 mg/dL). Placebo treatment produced no significant effect.
No serious adverse events occurred during the trial. Adverse events were reported for eight subjects (27%) given APHD-012 and five subjects (17%) given the placebo. The types of adverse events reported weren’t listed.
“I am highly impressed with the data,” said Christian Sina, professor of medicine at the University of Lübeck, Germany, and the trial’s principal investigator. “It marks the first instance of a drug demonstrating effectiveness in OGTT with a positive safety profile, which is crucial for a medication aimed at disease prevention. Preventing diabetes is a priority in healthcare programs due to the significant health and economic burden associated with this disease. Despite efforts in diet and exercise, effective therapies to prevent or delay the onset of type 2 diabetes are currently lacking, posing a challenge for individuals who do not respond to lifestyle changes. I eagerly anticipate supporting the next steps for the program, which has the potential to open a new avenue for prediabetes treatment.”
Aphaia Pharma is also running Phase 2 clinical trials to test the effectiveness of their oral glucose formulation as a means of chronic weight management in people with obesity.
Source: Aphaia Pharma