One of the ways scientists hope to rebuild muscles in patients where they are lacking is by taking cells responsible for entirely other functions in the human body and repurposing them. Known as direct cell reprogramming, scientists have now combined this technique with a new type of scaffold to ensure cells carry out their task following transplantation, and have demonstrated the potential of the technique by treating severe muscle loss in mice.
Though it is early days, reprogramming specific cells for other tasks in the human body is a technique with plenty of potential. We've previously seen how it could turn skin cells into beta cells that produce insulin for diabetics, turn structural cells into beating cells for damaged hearts and turn one type of eye cell into another to reverse blindness in mice.
In this new study, carried out by an international research team, the scientists have taken aim at what's known at volumetric muscle loss, which can result from serious injuries such as those sustained in car crashes or tumor removal. This leaves the sufferer without much of the muscles' natural ability to regenerate itself in the event of an injury, and current treatment options are limited.
The team set out to tackle this with the help of direct cell reprogramming, which entails turning one cell type into another without first returning it to an induced pluripotent state. This saw them enlist important structural cells in our connective tissue called fibroblasts, which were converted into induced myogenic progenitor cells (iMPCs) with the help of certain transcription factors.
The researchers then turned their attention to creating the support structure to ensure the transplanted cells would thrive in their new home. This led them to a biocompatible polyester called polycaprolactone (PCL), which was fashioned into a porous scaffold and mixed with a hydrogel containing a biomaterial commonly used to treat volumetric muscle loss, called decellularized muscle extracellular matrix.
Using this hybrid natural-synthetic scaffold in combination with their reprogrammed cells, the team produced engineered muscle fibers in vitro that exhibited properties similar to real muscle. Implanting these bionengeered muscle constructs into mouse models of volumetric muscle loss promoted muscle growth, innervation and the recovery of damaged muscles, effectively treating the condition.
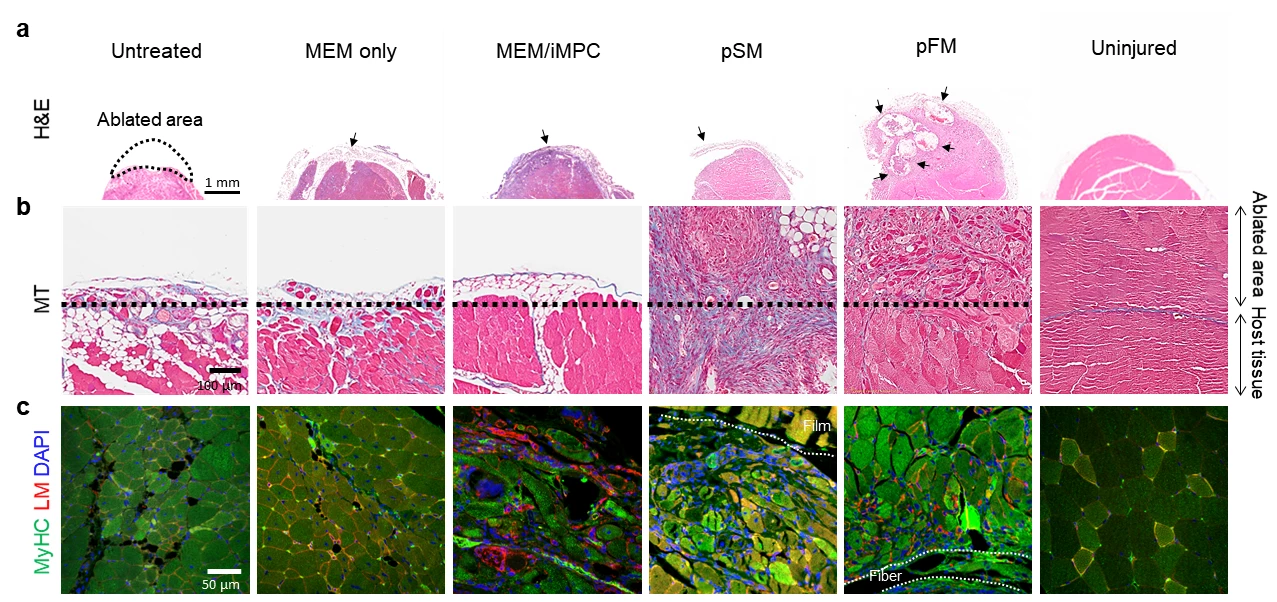
“Further studies are required to elucidate the mechanisms of muscle regeneration by our hybrid constructs and to empower the clinical translation of cell-instructive delivery platforms," says Professor Cho Seung-Woo from South Korea's Institute for Basic Science, who led the study.
The research was published in the journal Advanced Materials.
Source: Institute for Basic Science




