Scientists at the Department of Energy’s Lawrence Berkeley National Laboratory are using the 88-Inch Cyclotron to help steady the famous periodic table of elements one atom at a time where it's gone a bit wobbly at the heavy element end.
For many of us, the periodic table of elements is best remembered as a lifeline during a more than usually tedious science class. As the lesson droned on, the huge, inevitable poster on the classroom wall gave the students something arcane to look at and study as an alternative to dozing off and getting a tongue-lashing and possibly a piece of chalk tossed at your head.
Beyond that, the strangely laid out periodic table proved to be one of the most significant discoveries in the advancement in the history of science. First published in 1869 by Russian chemist Dmitri Mendeleev and later refined and revised by others, it marked the first successful attempt to properly organize the elements by their atomic weights and properties.
That in and of itself would have been a major achievement, but the remarkable thing about the periodic table was that not only did it reflect the nature of the elements, it showed that the elements were related to one another in such a way that chemists were able to predict their properties – even for elements that hadn't been discovered yet.
Because of the periodic table, chemists got a head start on a lot of ideas. For example, biochemists interested in lifeforms on other worlds could speculate that they might be made out of silicon or breathe chlorine because these elements are very similar to carbon and oxygen based on their places on the table.
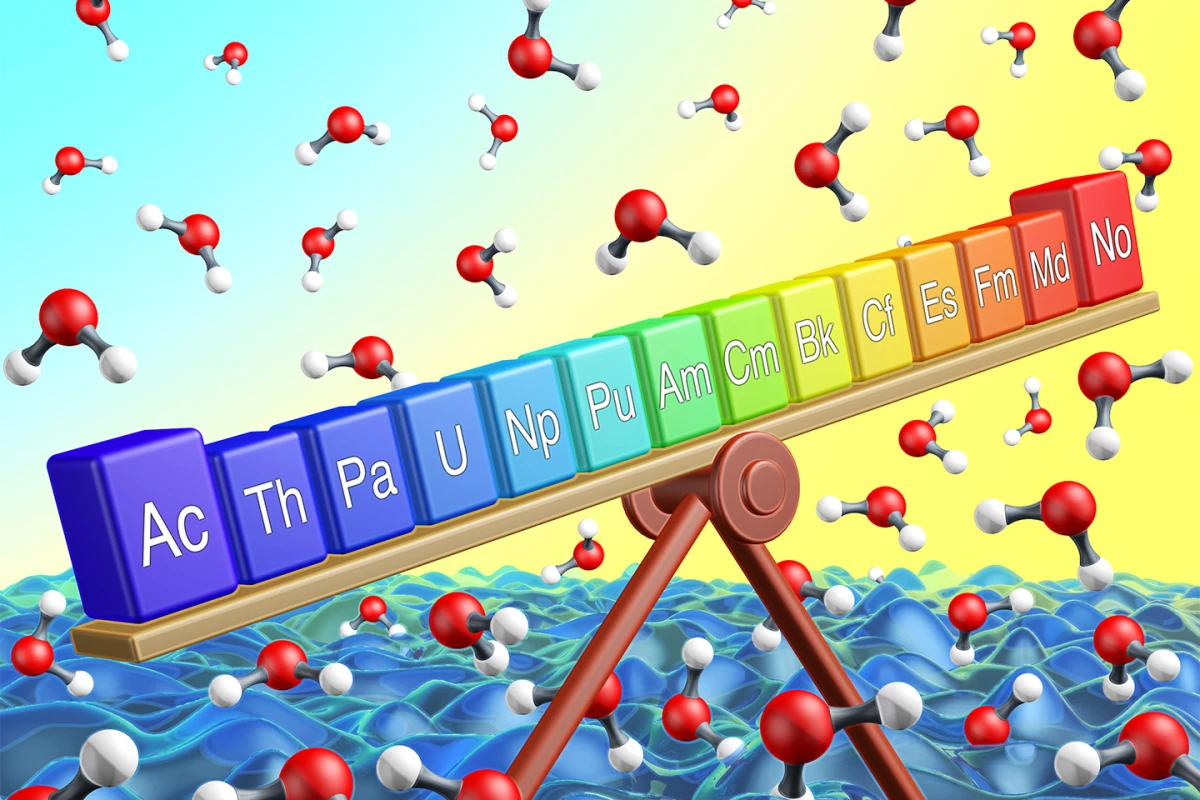
However, there is one snag. The periodic table works fine with the lighter elements, but when you start getting into the transuranics with an atomic weight of over 99, things start to break down and you can't predict properties with the same confidence.
This is because these atoms are so heavy that the negatively charged electrons are moving in orbits so large around the positively charged nucleus that they are traveling at speeds approaching that of the speed of light. The result is that Einstein's Theory of Relativity kicks in, causing the mass of the electrons to increase. This causes the orbits to contract, shifting the orbits of the other electrons, distorting their chemical properties.
Put it another way, when the atoms get really heavy, chemists have to rely on educated guesses on how these elements will interact with others to form molecules. This means that the only way to learn how the heavy elements behave is to study them and their molecules directly.
So, get out the test tubes and reagents? Not quite. These heavy transuranics are artificial elements that do not occur in nature and have to be made in a laboratory. This is because they are extremely unstable and therefore radioactive to an incredible degree. For example, element 102, Nobelium (No) is made up of isotopes with a half life measured in milliseconds, with the most stable lasting a mere 58 minutes before vanishing.
In other words, any chemical analysis has to be done very, very quickly. Mislay the filter paper and you're out of luck.
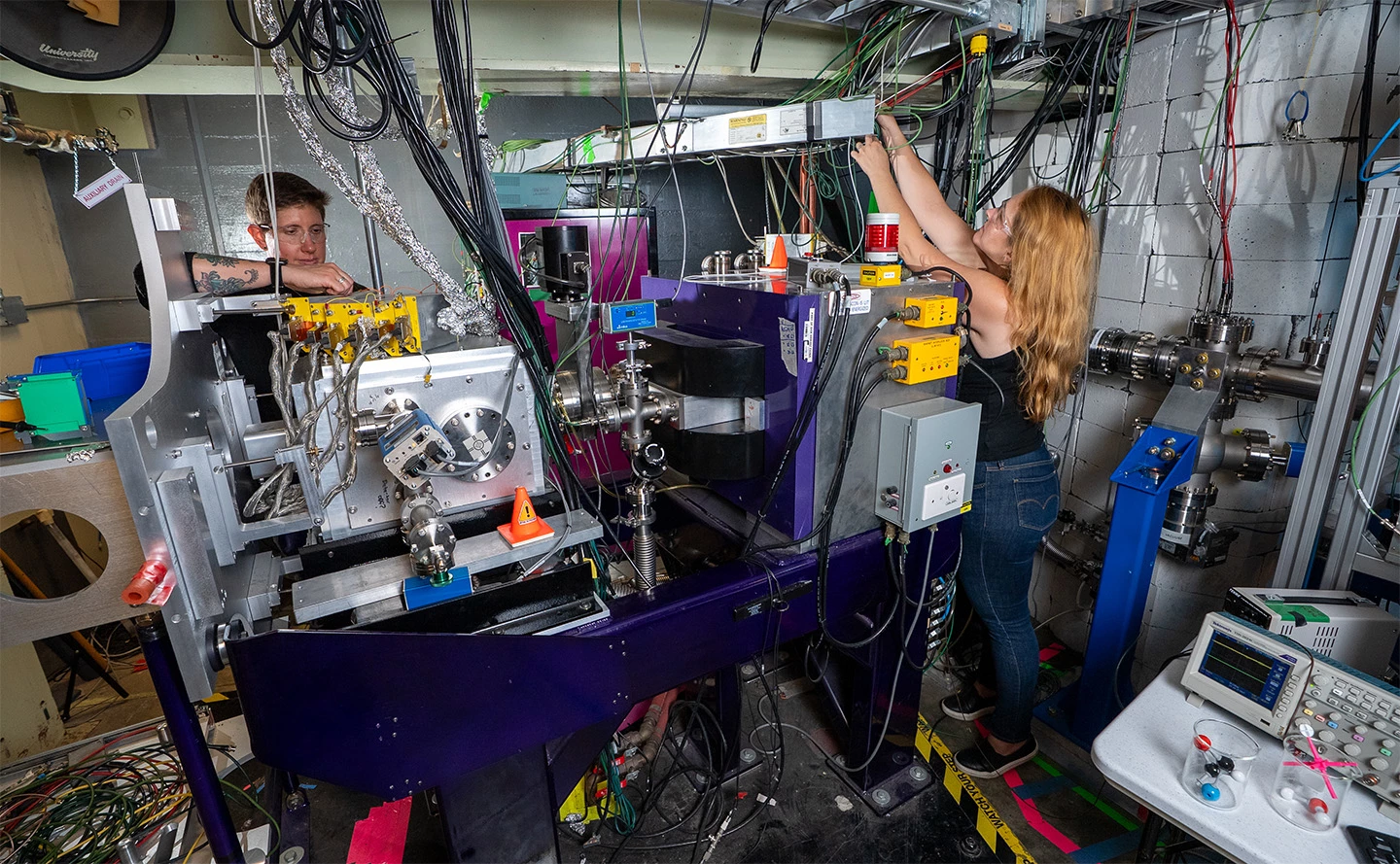
To overcome this, researchers at Berkley Lab dusted off a cyclotron first built in 1958 to study these elements one atom at a time as they form and before they decay. Set up with a mass spectrometer called FIONA, the apparatus can create heavy elements by firing a beam of calcium isotopes into a target of thulium and lead. This produces both nobelium and element 89, actinium (Ac), which is found at the opposite end of the same elemental group as nobelium on the periodic table.
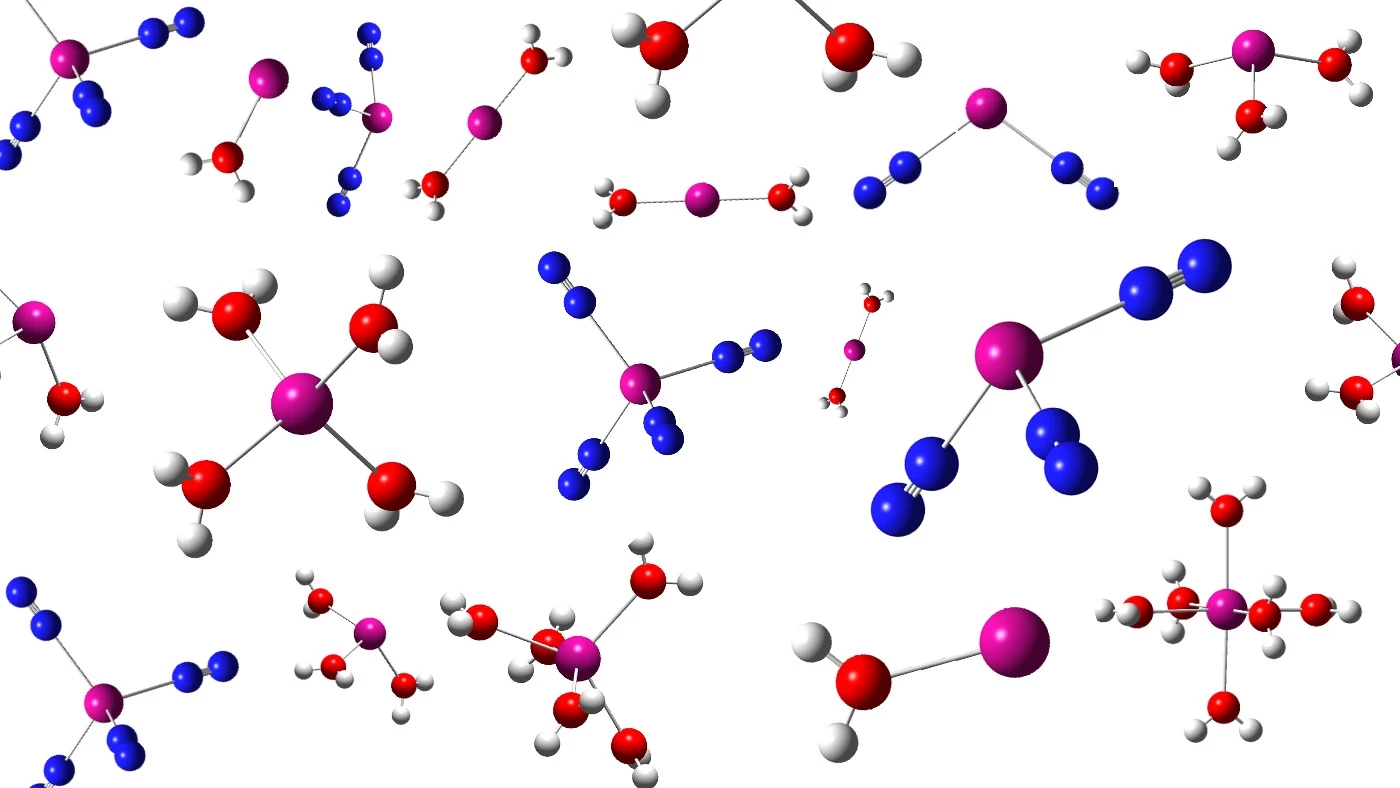
Once created, the scientists were surprised to find that the new atoms would spontaneously form molecules from the traces of water and nitrogen in the apparatus as they exited the 88-Inch Cyclotron at supersonic speeds. These molecules then went through FIONA, which analyzed them one atom at a time, directly measuring the molecules by their mass in milliseconds.
To give some idea of the scale involved, the experiment ran for 10 days and produced about 2,000 molecules. Contrast that with a drop of water, which contains 10²¹ molecules.
"What is really exciting is that this opens the door to the next generation of atom-at-a-time chemistry studies – so looking at the chemistry of superheavy elements and asking whether or not they are in the correct positions on the periodic table," said Jennifer Pore, a scientist at Berkeley Lab. "I think we’re going to completely change how superheavy-element chemistry is done."
The research was published in Nature.