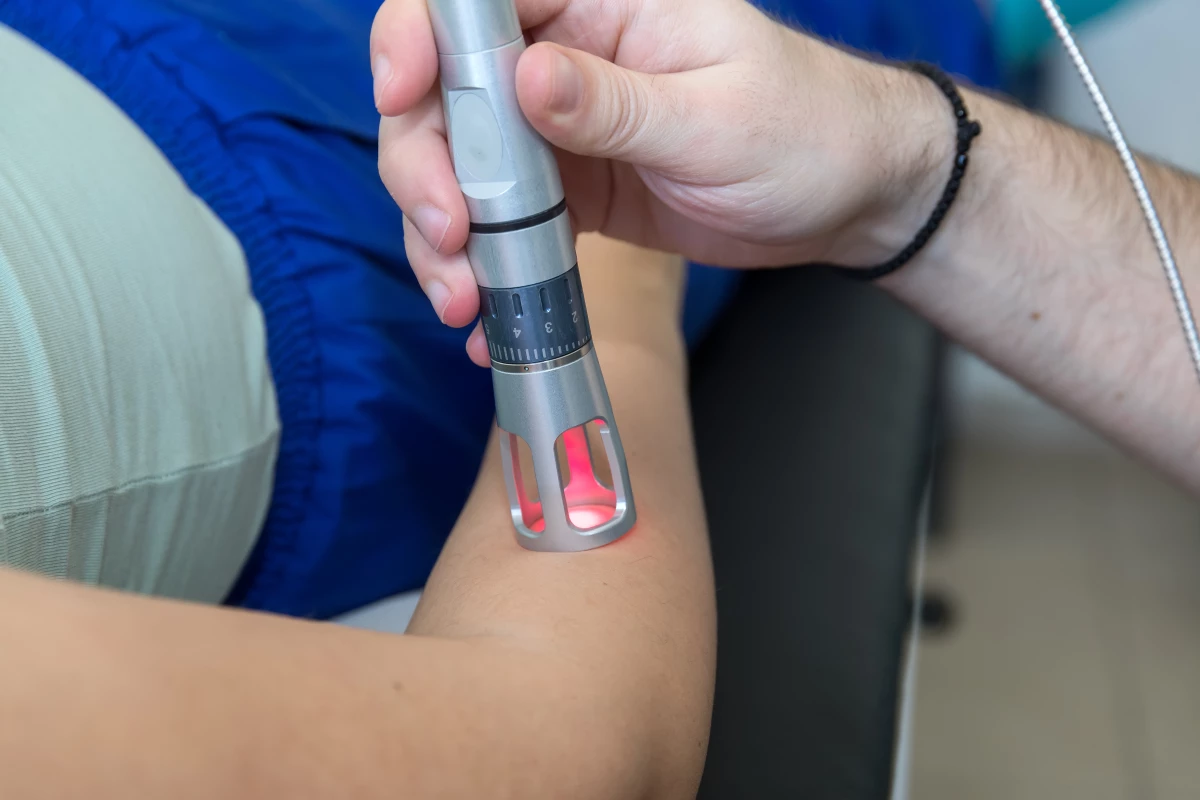
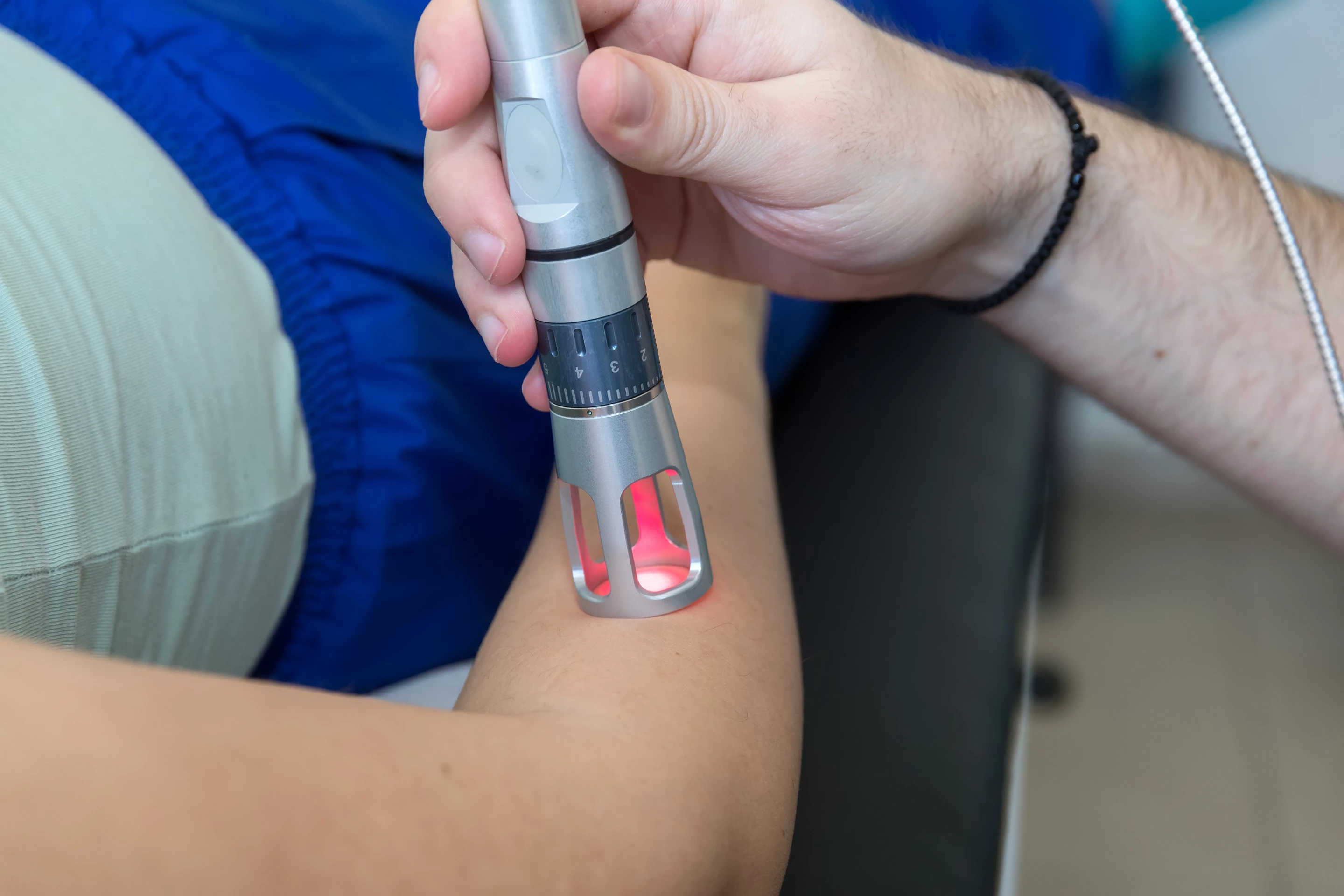
A compelling new study is offering an insight into the mechanism by which a novel form of light therapy can speed the healing of burn injuries. The research indicates the experimental therapy can activate a key protein that stimulates cell growth.
Low-level laser therapy, also known as photobiomodulation therapy, has been floated as a treatment for an assortment of maladies for around half a century. A review of the therapy in 2012 noted its effectiveness was still controversial. Decades of research has delivered relatively discordant results, and that may be due to two fundamental reasons.
“… first, its underlying biochemical mechanisms remain poorly understood, so its use is largely empirical,” the 2012 review explains. “Second, a large number of parameters such as the wavelength, fluence, power density, pulse structure, and timing of the applied light must be chosen for each treatment. A less than optimal choice of parameters can result in reduced effectiveness of the treatment, or even a negative therapeutic outcome.”
Using a series of cell and animal models, a new study published in the journal Scientific Reports set out to better understand the molecular mechanisms underlying photobiomodulation therapy. The particular focus was on burn wound healing and the effect light has on a protein called TGF‐beta 1.
TGF‐beta 1 is a protein that plays a fundamental role in several crucial cellular functions, including cell growth. The new research closely studied how light therapy influences TGF‐beta 1 activity in relation to a burn wound healing over nine days.
Alongside offering more evidence to show photobiomodulation therapy can speed up burn wound healing, the researchers demonstrated this process is indeed modulated by the enhanced activity of TGF‐beta 1. Praveen Arany, lead investigator on the new research, says the new findings indicate TGF‐beta 1 promotes wound healing by reducing local levels of tissue inflammation.
"Photobiomodulation therapy has been effectively used in supportive cancer care, age-related macular degeneration and Alzheimer's disease," notes Arany. "A common feature among these ailments is the central role of inflammation. This work provides evidence for the ability of photobiomodulation-activated TGF-beta 1 in mitigating the inflammation, while promoting tissue regeneration utilizing an elegant, transgenic burn wound model."
This particular study used a treatment protocol involving short bursts of low-level laser light at a frequency of 810 nanometers. Light was dynamically pulsed to maintain tissue surface temperature below 45 °C (113 °F).
The researchers hypothesize one of the key reasons for inconsistencies in prior studies could be target tissue being inadvertently heated up to temperatures that actually result in detrimental results. Several strategies are suggested for tests in the future to ensure photobiomodulation (PBM) does not warm tissue to unsafe levels.
“There are several successful strategies emphasizing the non-thermal nature of PBM protocols such as selecting a discrete wavelength such as visible light for superficial treatments and near-infrared for deeper targets, pulsing (increased thermal tissue relaxation), scanning delivery (small laser spot size) or large array (LED) patterns,” the researchers write. “Newer approaches such as surface tissue cooling, beam dose fractionation, up/down converting nanoparticles or photosensitizers and dynamic tissue temperature monitoring, as shown in this study, are also being effectively employed.”
Moving forward, the researchers do conclude this form of light therapy could be a valuable tool for wound management. But it seems a little more work is needed to better understand the safest and most effective way to administer this novel treatment before it can be confidently deployed in broad clinical uses.
The new study was published in the journal Scientific Reports.
Source: University at Buffalo