The connections between the brain's neurons are more malleable during the developmental stages of life, when the youthful organ continually reshapes the synapses between cells as we encounter new experiences, learn new skills and develop new habits. A great deal of research centers on restoring this youthful plasticity to older brains as a way of tackling some effects of aging, and a team in Austria has now identified a pair of promising new approaches, which involve specific flickers of light and high dosages of ketamine.
Efforts to equip older brains with the plasticity of juvenile ones are motivated by the hope that such a feat could open up new treatments for all kinds of neurological conditions. We've seen advances in this area involving molecular switches that boost memory and assist recovery from brain injury, neuron transplants that improve motor function, and gene therapies that restore visual capabilities in older mice.
In this new work, scientists at Austria's Institute of Science and Technology (IST) have focused on a structure that plays an important role in brain plasticity known as the perineuronal net, which we've also seen implicated in diabetes breakthroughs. This net encases some neurons in the brain, locking in the existing connections between them and preventing new ones from forming.
In this way, the perineuronal net is key to the storage of childhood memories and habits, but by the same token, stops a mature brain from being as adaptable as a youthful one. The scientists sought to explore whether the net could be temporarily dismantled, making neurons more receptive to new experiences and allow new synapses, or connections, to form.
Their discovery begins with mice that had been anesthetized with ketamine, which the scientists found caused cells in their brains called microglia to become highly reactive. In this state, microglia have the capacity to eat up synapses and neurons, something seen in late-stage Alzheimer's, but the scientists saw no evidence of this taking place.
“The strong response of the microglia upon ketamine anesthesia surprised us,” explains Alessandro Venturino, lead author of the study. “But we did not see any synapses or dead neurons vanishing. So, we were puzzled, what the microglia were actually eating.”
To their great surprise, the scientists found that the microglia were actually gobbling up the perineuronal net.
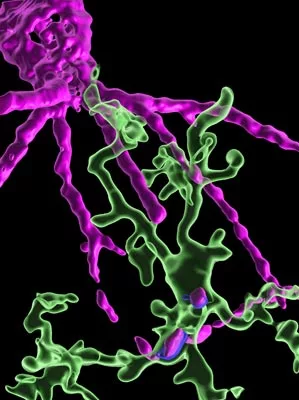
“Alessandro came to my office and told me that the perineuronal net was gone. I could not believe it,” says leader of the research group Sandra Siegert. “After just three treatments, we could see a considerable loss in the perineuronal net, which lasted for seven days before being rebuilt.”
The prospect of using non-invasive ketamine treatment to induce temporary changes to the perineuronal net is an exciting one, as techniques that remove the net have been demonstrated before, but with long-lasting effects and only through highly invasive measures.
But through their experiments, the scientists may have uncovered another, even less invasive approach, in flickering light.
This type of research looks to leverage the way neurons communicate, by passing coordinated electric impulses between one another. By using external stimuli, such as a light flickering at a specific frequency, the idea is that these signals can be manipulated for better health outcomes. One application with lots of potential concerns Alzheimer's, with one 2019 study showing how a light flickering at 40 Hz can boost microglia and help clear away toxic proteins associated with the disease.
Subjecting mice to light flickering at 40 Hz in the experiments was found to have no effect on the perineuronal net. But when the rodents were placed in boxes with a light flickering at 60 Hz, the scientists found it induced a similar effect on the structure to the ketamine treatments.
“It had been previously shown that light flickering 40 times a second – at 40 hertz – can promote microglia to remove plaques in Alzheimer disease," says Venturino. "But it did not remove the perineuronal net. This fine-tuning between distinct brainwaves and the microglia action is the most fascinating and might be a new way of thinking about brainwaves.”
These discoveries open up two promising new avenues in potentially restoring youthful plasticity in human brains. The researchers note that there is plenty still to learn about how these techniques work, but imagine they could one day be used to treat conditions like lazy eye, caused by an unbalanced visual input during childhood, or possibly overwrite painful memories and experiences to treat post-traumatic stress disorder (PTSD). At the same time, they are very aware of the risks such an approach could pose.
“But we are very cautious because in this formative window also something traumatic could happen,” Siegert says. “It is probably also not a good idea to blast yourself with flickering light.”
The research was published in the journal Cell Reports.




