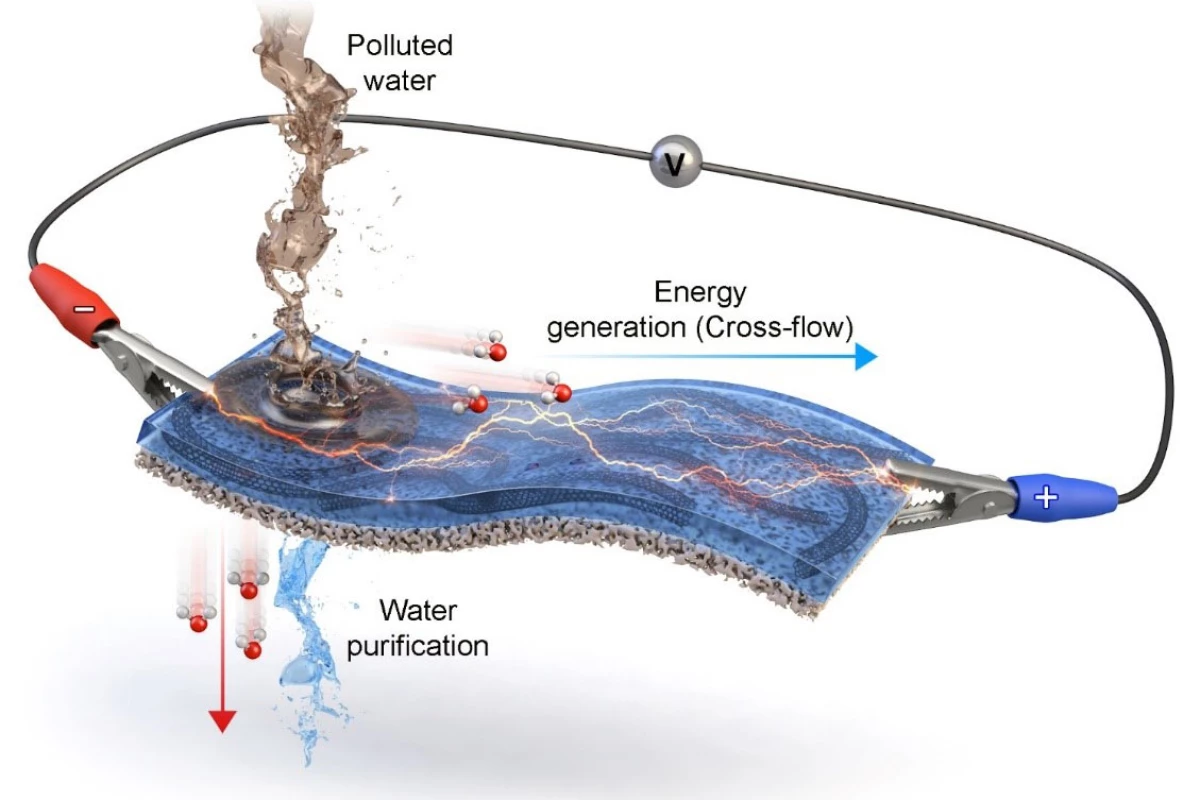
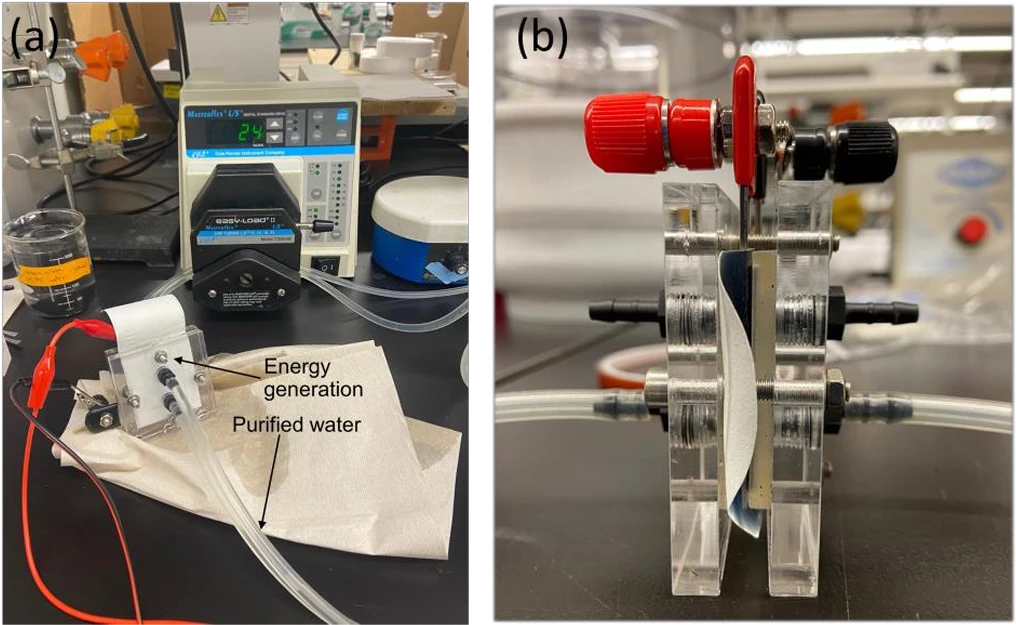
A team from the Korea Institute of Science and Technology (KIST) and Myongji University, both located in Seoul, has published a new paper describing an "electricity generation and purification membrane for water recycling."
The team claims it can reject more than 95% of contaminants smaller than one hundred millionth of a meter in size, including heavy metal particles and the microplastics that are now found in distressing quantities in rainwater from Antarctica to the Tibetan Plateau, rendering it unsafe to drink. It seems to work regardless of the acidity of the water source as well, performing well across a pH range of 1-10.
The membrane is a two-layer sandwich, the top layer being a conductive polymer, and the bottom being a porous filter. As contaminated water is poured onto the top layer, it moves laterally across the membrane, creating a cross-flow of ions that can be harvested as an electrical current using electrodes at either end of the membrane.
While the study claims the membrane showed "high energy generation performance" in experimental testing, the lab prototype is small and so are the corresponding power figures. The study abstract reports a maximum power level of just 16.44 microwatts, and a maximum of 15.16 millijoules of energy generated over an unspecified time period. And the power generation is continuous – just 10 microliters of water was enough to generate electricity for more than three hours.
The team is now working on follow-up research to scale up its work to factory-relevant size, a press release claiming that "since the membrane can be manufactured using a simple printing process without size restrictions, it has a high potential to be commercialized due to low manufacturing costs and processing time."

Lead author Ji-Soo Sang sees the material having potential as a next-generation renewable energy source. "As a novel technology that can solve water shortage problem and produce eco-friendly energy simultaneously," he says, "it also has great potential applications in the water quality management system and emergency power system."
The paper is published in the journal Advanced Materials.
Source: KIST