There may be a new way of keeping orange and grapefruit peels out of our landfills – besides composting them, that is. Researchers have devised a method of using the peels to remove heavy metals from wastewater.
The research was carried out by scientists at Spain's University of Granada, along with colleagues at Mexico's Center for Electrochemical Research and Technological Development, and Center of Engineering and Industrial Development.
First, the peels are desiccated using a vacuum drying treatment, after which they're subjected to a proprietary chemical treatment. As a result, their porosity and surface area increases. They're then granulated.
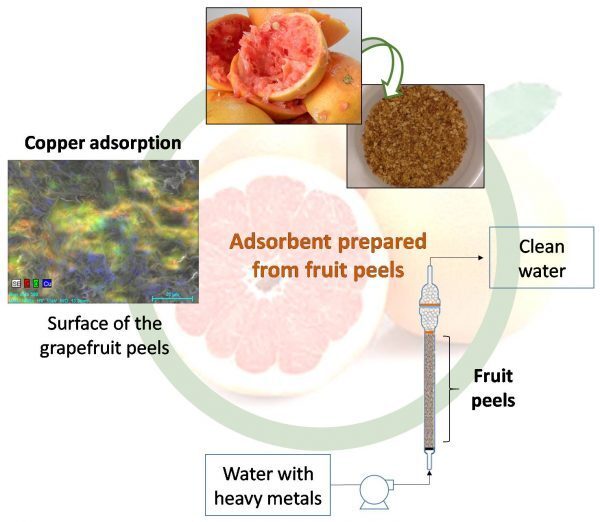
In lab tests, the treated peels were placed in columns (see diagram above) where they successfully adsorbed heavy metals such as copper from tainted water that was pumped through.
"The results show a great potential for the use of said materials as adsorbents capable of competing with commercial activated carbon for the adsorption and recovery of metals present in wastewater," says Romero Cano, a team member from the University of Granada. "It could be possible to carry out sustainable processes in which products with a great commercial value could be obtained from food industry residues."
A paper on the research was recently published in the journal Industrial Crops and Products. Scientists at Australia's Flinders University have also been removing heavy metals from water, using a polymer that incorporates a substance obtained from citrus peels.
Source: University of Granada





