In 2022, scientists examined the brains of 22 stranded marine mammals and found something astonishing: Alzheimer's-like pathology in dolphins. And not just vague signs, actual amyloid plaques and tau tangles, the same hallmarks seen in human patients.
Now, a new study has doubled down on the discovery, revealing stranded dolphins showed brain damage eerily similar to that of people with Alzheimer's. Just as people with dementia sometimes wander far from home, scientists think dolphins with Alzheimer's might get confused at sea. It's the strongest evidence yet that neurodegeneration isn't just a human affliction; it may ripple through the animal kingdom, too.
But perhaps most significantly, these changes may be linked to cyanobacteria, toxic microbes often found in polluted waters.
In Florida's Indian River Lagoon (IRL), climate change and pollution fuel toxic algal blooms. These blooms release toxins that accumulate in the food chain, posing a threat to fish, land animals, and even humans. Dolphins, being long-lived and highly exposed, can serve as nature's early warning system. Their brains and bodies reveal what chronic toxin exposure might mean for all of us.
Scientists studied 20 common bottlenose dolphins (Tursiops truncatus truncatus) that were stranded in the IRL, using Triple quadrupole tandem mass spectrometry (QqQ or TQMS). It is a razor-sharp analytical technique that can zoom in on specific compounds, even in messy biological or environmental samples.
In the IRL, dolphin deaths tend to spike during the summer, right when harmful algal blooms (HABs) are at their worst. Scientists believe this seasonal surge isn't a coincidence. Instead, it points to a troubling pattern. Warmer months may heighten dolphins' vulnerability to neurotoxic algal compounds.
The study shows a startling pattern. During HAB seasons, dolphin brains were found to contain 2,900 times more 2,4-diaminobutyric acid (2,4-DAB) or DABA – a sneaky neurotoxin made by cyanobacteria, diatoms, and dinoflagellates – than in non-bloom periods.
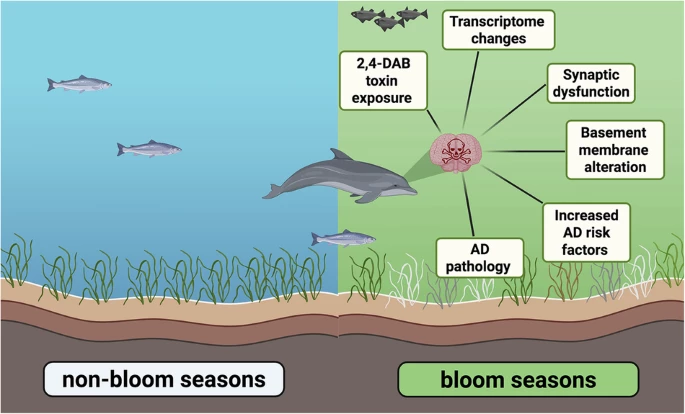
Once labeled a neurolathyrogen, a compound that damages nerves, DABA doesn't waste time. Within hours of exposure, it can trigger hyperirritability, tremors, and convulsions. That's because it acts like an excitatory amino acid, overstimulating brain cells by messing with their electrical balance.
But the damage runs deeper. These dolphins exhibited 536 altered genes, indicating disrupted GABAergic synapses, changes in the basement membrane, and increased Alzheimer's risk factors, which worsen with each bloom season.
The team also found that dolphins stranded during bloom seasons had lower levels of glutamate decarboxylase (GAD). This crucial enzyme transforms glutamate (a stimulating neurotransmitter) into GABA (a calming one). Without enough GAD, the brain's balance tips toward overexcitation, leading to dyskinesia, mental, psychiatric, neurodegenerative, and neurodevelopmental disorders.
In Alzheimer's disease, both GAD1 and GAD2 genes are known to decline. The neurotoxin DABA may be accelerating this decline, deepening the damage, and pushing dolphin brains further into dysfunction.
The study revealed that uneven seasonal exposure to 2,4-DAB amplifies Alzheimer's disease-related transcriptomic signatures in the brain. With climate change driving the intensification of HABs, the researchers emphasized the importance of investigating how such environmental exposures may contribute to neurological risk, particularly among vulnerable populations.
Scientists also found that at least half of stranded bottlenose dolphins suffered severe to profound hearing loss. This condition is known to affect behavior, navigation, and social bonding. While this particular study didn't include hearing data for its dolphin cohort, the team uncovered something intriguing: gene transcripts (MYO1F, STRC, SYNE4), all linked to hearing, were correlated with 2,4-DAB exposure, bloom season, and year of stranding.
The authors noted that in humans, hearing loss also serves as a risk factor for Alzheimer's disease (AD) and may accelerate AD-related neuropathology.
They emphasized that, given the observed effects of 2,4-DAB on the brain transcriptome, further research is necessary to elucidate the role of this molecule in the development of neurodegenerative diseases.
The study was published in the journal Communications Biology.
Source: Brain Chemistry Labs





