Researchers have discovered new information about how the body’s immune system works, simultaneously calling into question decades of understanding about the process of antibody-mediated immunity and opening the door for advancements in immunological treatment.
B cells, a type of white blood cell, drive the body’s antibody-mediated immunity, creating antibodies that bind to pathogens or foreign substances and neutralizing them. B cells can also produce harmful antibodies that attack normal tissue, causing allergic reactions and autoimmune diseases.
Antibodies are made in response to antigens, markers that tell your immune system when something foreign – bacteria, a virus, or vaccination – is present in your body. The antibodies recognize and attack harmful antigens, but they are particular. Antibodies are like a key, and an antigen is a lock; antibodies will only unlock specific antigens.
Embedded in the B cell’s surface is a B-cell receptor (BCR), a protein that stands guard, ready to bind with antigens. For decades, scientists and researchers thought an antigen would cross-bind with several BCRs, grouping signal molecules together and transmitting the signal into the cell, which attracted help from T cells to destroy the invader.
The illustration below shows two antigens (light blue spheres) bound to a cluster of BCRs (purple, Y-shaped structures) on the cell’s surface. The dark blue and black shapes represent signal molecules transmitting the signal into the cell (lightning bolt).
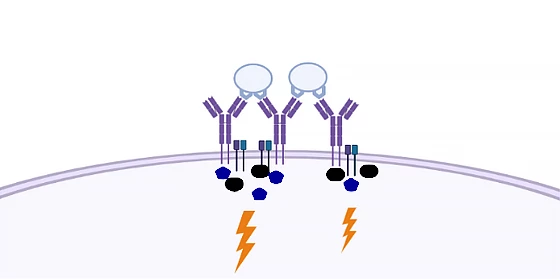
But researchers from Aarhus University in Denmark, in collaboration with the Max Planck Institute in Munich, have turned years of scientific consensus on its head with a new study into the molecular mechanisms underpinning B cell activation.
Their research sought to answer two questions. First, when a cell is in its resting state, how are BCRs distributed? And second, what are the minimal requirements for antigen-driven activation of a BCR?
To answer the first question, the researchers used DNA-based point accumulation for imaging in nanoscale topography (DNA-PAINT). This super-resolution method allows single-molecule visualization of nucleic acid nanostructures to a resolution of around five to 10 nanometers. They found that when the cell is in a resting state, BCRs are not clustered in an organized way, as had previously been thought.
Addressing the second question, the researchers examined the precise ratio of antigens to BCRs. Finding no qualitative difference between activation by single and multiple antigens, they concluded that single antigens could activate B cells.
The study’s findings are illustrated below, where singular, spaced-out BCRs interact with antigens (here, light blue hexagons) to elicit the same signaling response in the cell.
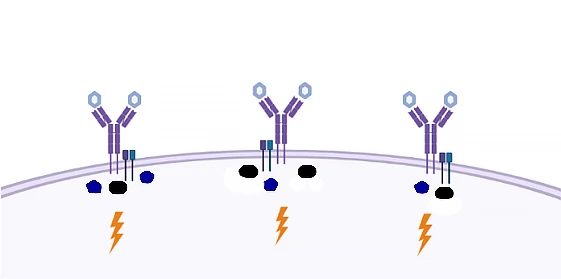
“We have shown that the way in which the activation of B cells has been explained over the past 30 or 40 years is wrong,” said Associate Professor Søren Degn, corresponding author of the study. “This is an important finding, because it opens the door to better vaccines and better treatment of a large group of diseases.”
Uncovering the biological process underlying the body’s B-cell-mediated immune response is an important discovery with potentially widespread ramifications.
“The result is significant because it represents a breakthrough in our understanding of how these important immune cells ‘recognize’ their enemies,” Degn said. “When we understand how the B cells are activated, we can create better vaccines. In the slightly longer term, we may also be able to switch off B cell activation in cases where it is harmful.”
The study was published in the journal Nature Communications.
Source: Aarhus University




