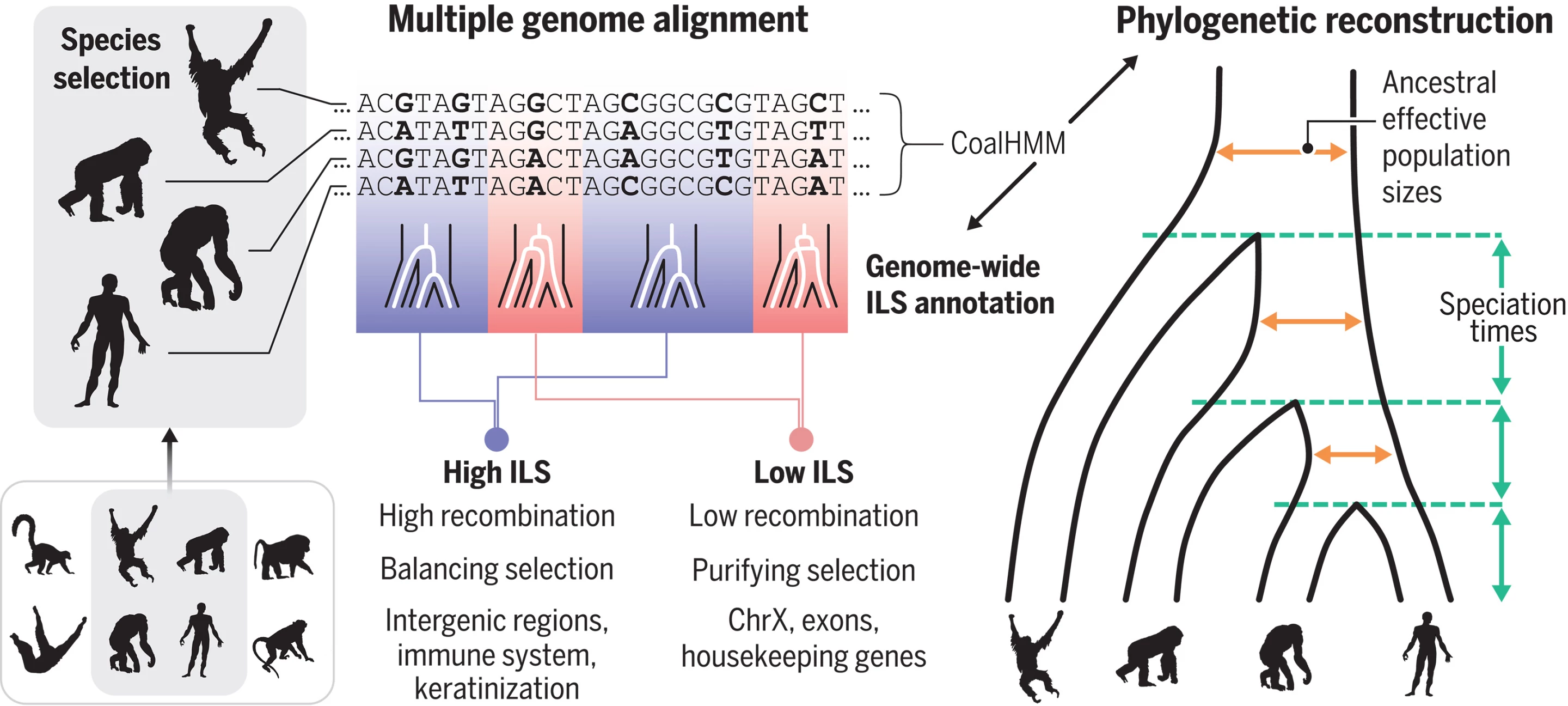
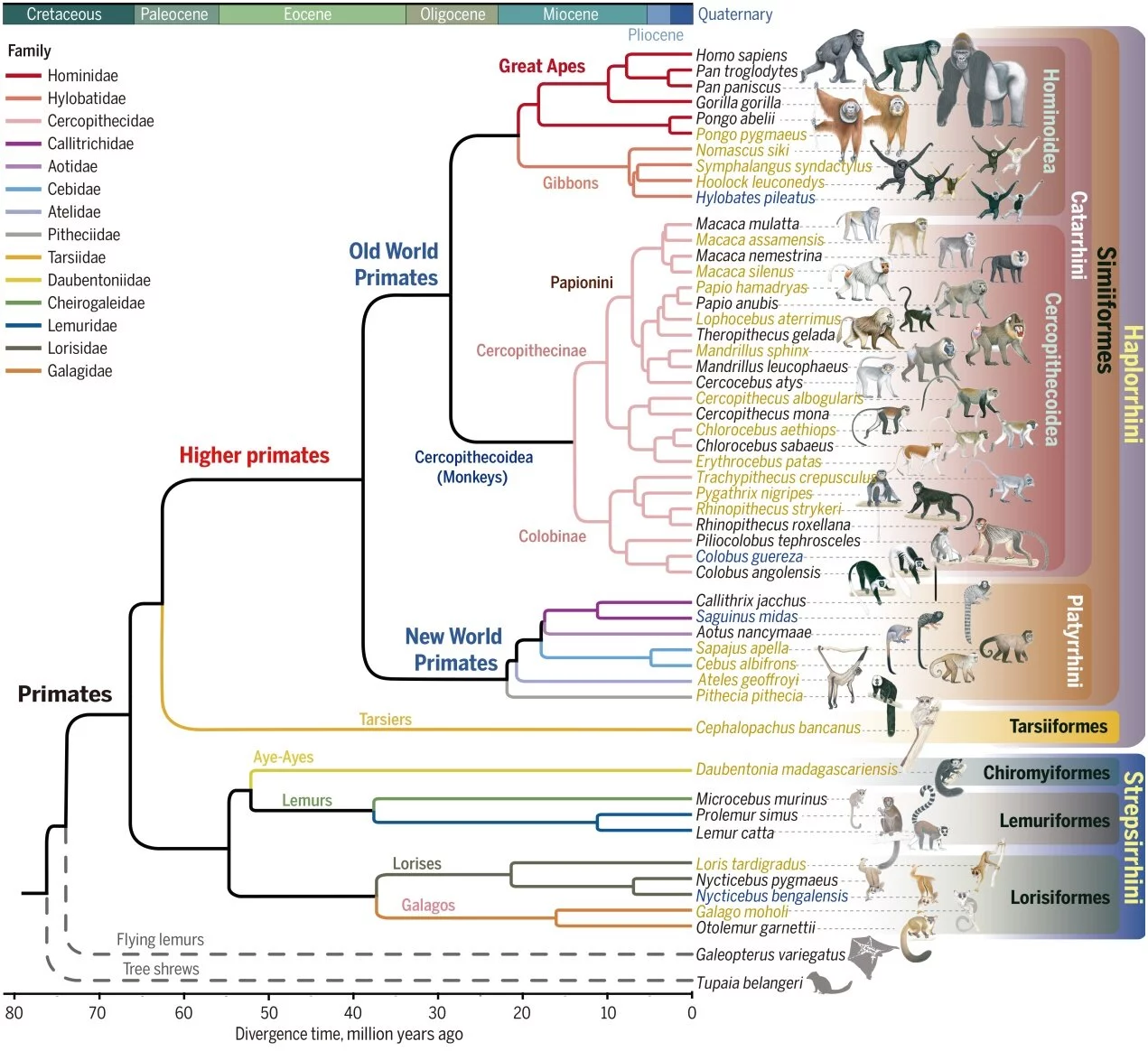
In a world first, scientists from 24 countries have mapped the DNA of more than 233 different primate species, more than quadrupling the existing genetic data, providing crucial new insights into disease-causing genetic mutations in humans.
“Humans are primates," said lead author Tomàs Marquès-Bonet, professor at Pompeu Fabra University, Spain. "The study of hundreds of nonhuman primate genomes, given their phylogenetic position, is very valuable for human evolutionary studies, to better understand the human genome and the bases of our singularity, including the bases of human diseases, and for their future conservation."
In one study, researchers used the 233 primate blueprints to inform a new artificial intelligence tool, which accurately identified and isolated genetic variations in human genomes responsible for diseases.
"Because of their closeness to the human genome, nonhuman primate species are uniquely valuable, both for what they can teach us about the genetic basis of human diseases, and in their own right," said senior author Kyle Farh, vice president of Artificial Intelligence at biotech research company Illumina.
“We discovered that if a ‘rare’ mutation cannot be found in the primate genome, it is very likely to cause a human disease,” said Farh. “In addition, some of these rare mutations can cause, by themselves, some diseases considered polygenic.”
According to the National Institutes of Health, 40 billion gigabytes of genomic data is now being generated each year, with the figure only set to increase. But it’s of little use without the tools that can cheaply and quickly analyze and interpret it.
PrimateAI-3D, not unlike ChatGPT but instead using a system of natural selection for learning, features a neural network of millions of benign genetic variants from the 233 species. In the study, it was able to identify disease-causing genetic variants in six human cohorts tested and was able to also accurately provide personalized predictions of genetic disease risk in a study of nearly half a million human genomes from the UK Biobank.
"The application of the latest advances in AI to genomics opens tremendous opportunities for Illumina in both genetic risk prediction and drug target discovery by decoding the basis of complex genetic diseases such as diabetes, heart disease, and autoimmune diseases," said Alex Aravanis, chief technology officer of Illumina.
The Primate Genome Project, which looked at 809 individual animals across all 16 families of primates, is the most ambitious and comprehensive DNA database to date and covers nearly half of all living primate species.
The data has also thrown a spanner in the works of what we believed separated us from our ancestors. Researchers discovered that many species had much more shared DNA with humans than previously thought, halving the genetic catalogue thought to be exclusive to Homo sapiens.
“These studies bring comparative genomics to new heights, and we can predict the impact on both understanding of human biology and on practical clinical diagnostic issues,” said Dr Richard Gibbs, founding director of the Human Genome Sequencing Center and Wofford Cain Chair and Professor of Molecular and Human Genetics at Baylor.
“When we investigate the genomics of nonhuman primates, we not only learn about these species, which is important and timely, but we can also place human genetics into its proper comparative context, which provides new insights into human health and human evolution,” added Jeffrey Rogers, lead investigator and associate professor at the Human Genome Sequencing Center at Baylor.
The research was published in a special edition of the journal Science.
Sources: Baylor College of Medicine, Illumina