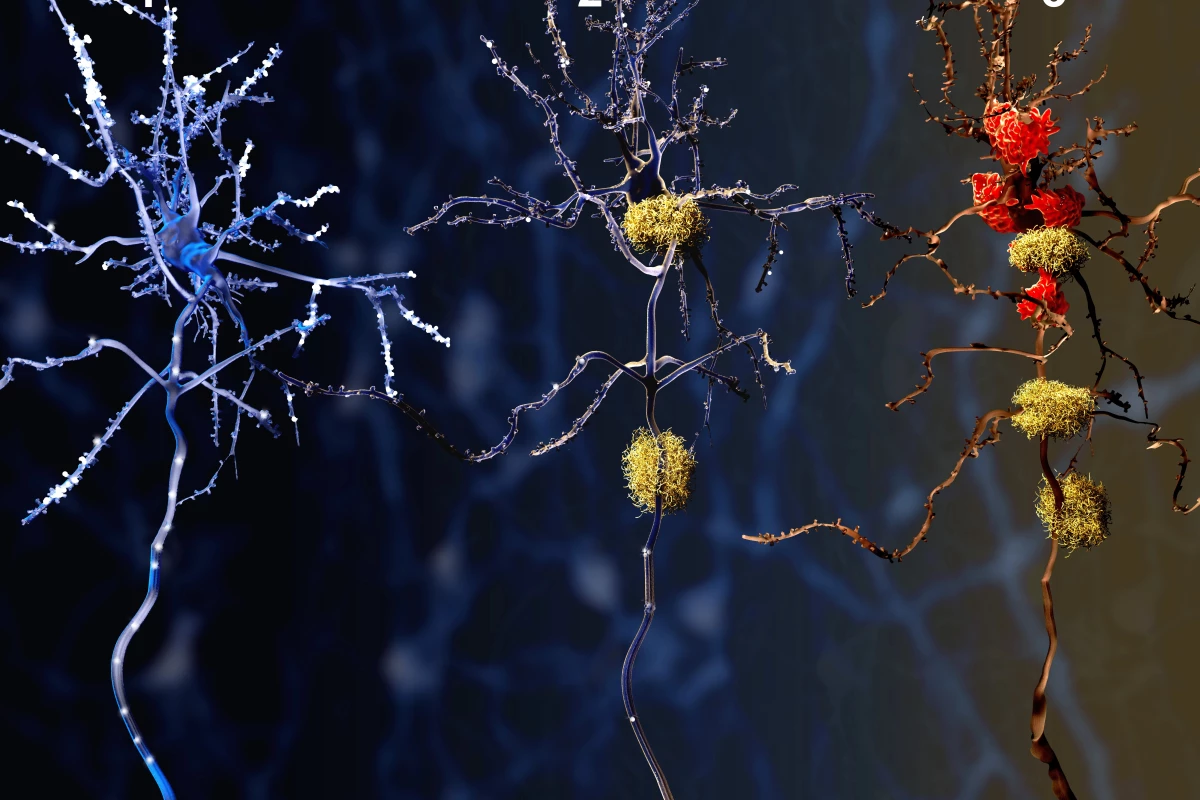
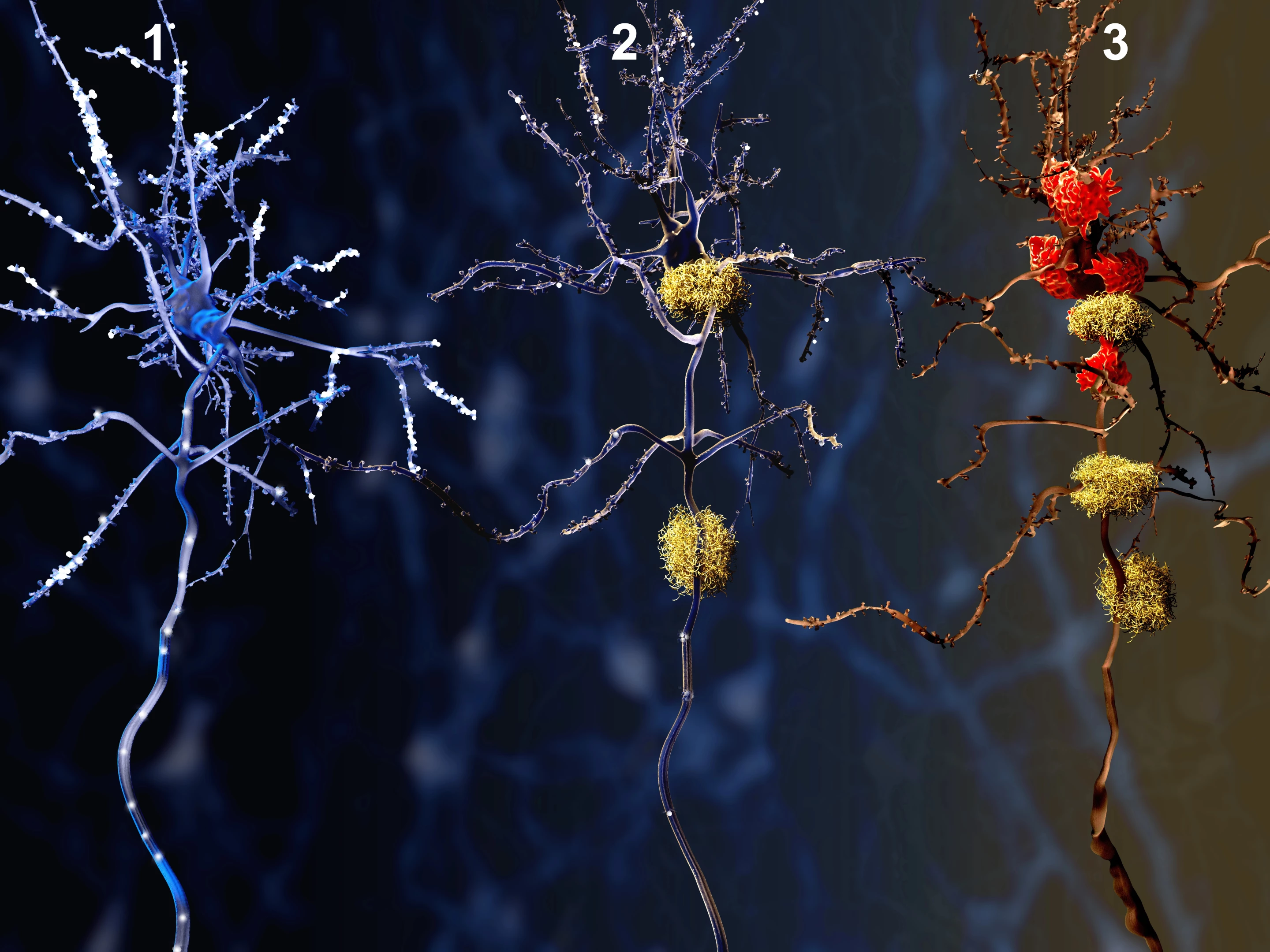
Taking inspiration from the CAR T-cell technology used to provide personalized cancer treatments, researchers have conducted a proof-of-concept study showing how similar compounds can precisely target protein tangles and plaques in the brain.
While not the cause of Alzheimer's, these protein build ups are certainly the disease's most prominent hallmarks. They are also responsible for the cognitive decline seen with the condition because they gunk up the workings between neurons in the brain.
While current anti-Alzheimer's drugs target the plaques and tangles, they can also have serious side effects.
“The current issues with FDA-approved drugs for Alzheimer’s, including side effects such as brain bleeds and seizures, highlight the desperate need for targeted treatments that would leave the brain generally unscathed,” says senior study author Julie Andersen. “Current treatments act as a sledgehammer. We aim to develop a targeted scalpel.”
To see if they could do better, Andersen and a team of researchers at the Buck Institute for Research on Aging in California used the work that's been done developing anti-cancer CAR T-cell therapy as a jumping off point. That therapy works by engineering a patient's own disease-fighting T cells to produce a protein known as chimeric antigen receptor (CAR), which then allows them to identify, bind to, and destroy specific cancer cells.
Biological autonomous taxi
For their study, the Buck researchers built a set of CARs using portions of Alzheimer's antibodies, some of which are currently being tested in phase III clinical trials. When they introduced the CARs to mice with Alzheimer's disease, they observed them easily binding to tau and amyloid beta proteins in their brains. This means that eventually, the antibodies could be equipped with medication to dissolve those compounds and fight the progression of the disease.
"Protein tangles and plaques have been the focus of Alzheimer’s treatments for decades,” says lead researcher Chaska Walton. “What we’re showing for the first time is that immune cells can be trained to recognize not just amyloid or tau in general – but specific forms of these proteins that are thought to be most toxic. It’s a bit like an autonomous taxi – you type in the destination address and the engineered receptor cells end up exactly where you want them to be.”
Saving, not killing
Moving forward, the team feels that its work will follow the same path that CAR T-cell therapy followed for cancer, with one important distinction.
“It’s important to note that this technology does not involve the same toxicity seen in CAR-T cells,” said Walton. "Those receptor cells are designed to kill cancer cells. Our cells will be designed to heal. We want to save neurons.”
Next will come arming the CAR proteins with a therapeutic payload and checking their efficacy in mice engineered to have Alzheimer's. Then if all goes well, the concept could move into human testing. The researchers are encouraging the adoption of their methods – perhaps even beyond treating Alzheimer's – by freely sharing their results.
“A lot of researchers will test their constructs and report out results, but they won't give you the complete amino acid sequences to make them," concludes Walton. "You really have to dig through the scientific literature and patents to figure them out, and even then, some just aren’t available. But the sequences are in our study. They're there and anyone could use them for their cells, whatever cell type they are, which is the way research should be.”
The research has been published in the Journal of Translational Medicine.