A clinical trial has shown that Ozempic improves blood glucose levels and weight loss in overweight type 1 diabetics who use an automated insulin delivery system. It’s hoped that this will lead to the approval of Ozempic as an adjunct therapy for this population of diabetics.
There’s no doubt that the drug semaglutide (Ozempic) has revolutionized the treatment of type 2 diabetes. Used together with diet and exercise, the drug has been shown to provide better blood glucose control and lower the risk of heart attack, stroke, or death.
Now, a clinical trial led by the Indiana University (IU) School of Medicine has shown that semaglutide improved blood glucose levels and weight loss in obese type 1 diabetics who use an automated insulin delivery (AID) system – a combination of an insulin pump and continuous glucose monitoring (CGM).
“We found that semaglutide was effective in improving time spent in the target blood sugar range and reduction in body weight compared to placebo group,” said Viral Shah, MD, the study’s lead author and professor of medicine at IU.
Type 1 and type 2 diabetes both affect how the body handles blood glucose, but they’re quite different in cause and treatment. Type 1 diabetes (T1D) is an autoimmune condition, usually diagnosed in children or young adults, where the immune system mistakenly destroys the cells in the pancreas that produce the glucose-lowering hormone, insulin.
Type 2 diabetes (T2D), on the other hand, usually develops later in life and is linked to lifestyle and genetic factors. In this case, the body still makes insulin but doesn’t use it properly, leading to high blood sugars (hyperglycemia). While T2D can often be managed with diet, exercise and medication, T1D requires insulin from the start and for life.
Seventy-two adults with T1D on an AID and a body mass index of 30 or above (the definition of “obese”) were randomly assigned to two groups. For 26 weeks, one received a once-weekly shot of semaglutide, while the other was given a placebo. The primary outcome of the trial was to achieve all three of the following:
- Spend more than 70% of the day with a blood glucose in the target range of 70–180 mg/dL (3.9–10.0 mmol/L).
- Spend less than 4% of the day with low blood glucose or hypoglycemia, that is, a glucose reading below 70 mg/dL (3.9 mmol/L).
- A reduction in body weight of 5% or more.
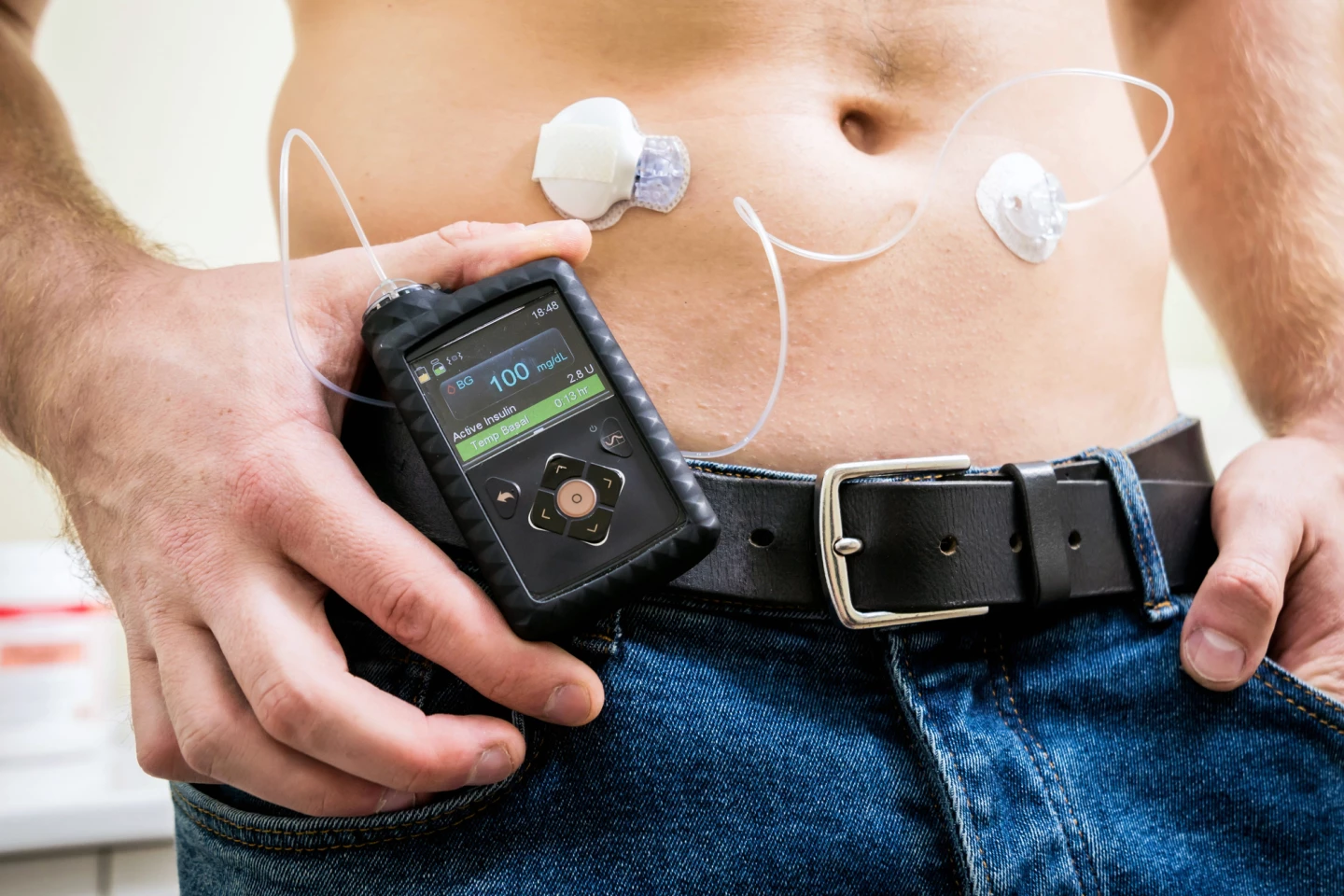
Among the trial participants on semaglutide, 36% hit all three goals. None of the participants in the placebo group did. On average, semaglutide users lost 19.4 lb (8.8 kg) more than placebo users. They also spent more time in the healthy blood sugar range and had lower average HbA1c, which is a measure of long-term blood sugar control. The researchers noted no increase in dangerous complications such as diabetic ketoacidosis (DKA), a life-threatening complication of diabetes that occurs when there’s a lack of insulin and the body has to burn fat for energy instead of glucose, leading to a buildup of ketones in the blood. There were only two cases of severe hypoglycemia (low blood glucose) in each treatment group.
The trial provides the first evidence that semaglutide may also help type 1 diabetics better control their condition, thereby reducing the risk of complications. Adding semaglutide to existing insulin therapy can lead to better blood sugar management and significant weight loss, which may well be a game-changer for people with T1D who also struggle with obesity. This group typically has few options beyond insulin.
There are a couple of caveats. First, it was a small cohort of only 72 people; larger trials are needed before broad recommendations can be made about approving semaglutide for use by type 1 diabetics. Second, the trial only considered diabetics using an automated insulin delivery system, so the results don’t apply to all type 1 diabetics. Nonetheless, the researchers are hopeful.
“We hope that our trial will encourage the industry to conduct a regulatory approval trial so that this drug could be available as an adjunct to insulin therapy to optimize type 1 diabetes management,” said Shah.
The study was published in the journal NEJM Evidence.
Source: Indiana University







