A once-daily pill for ulcerative colitis has delivered strong results in a clinical trial, easing symptoms even in patients for whom other treatments had failed and raising hopes for a safer, more effective therapy for a condition that affects millions.
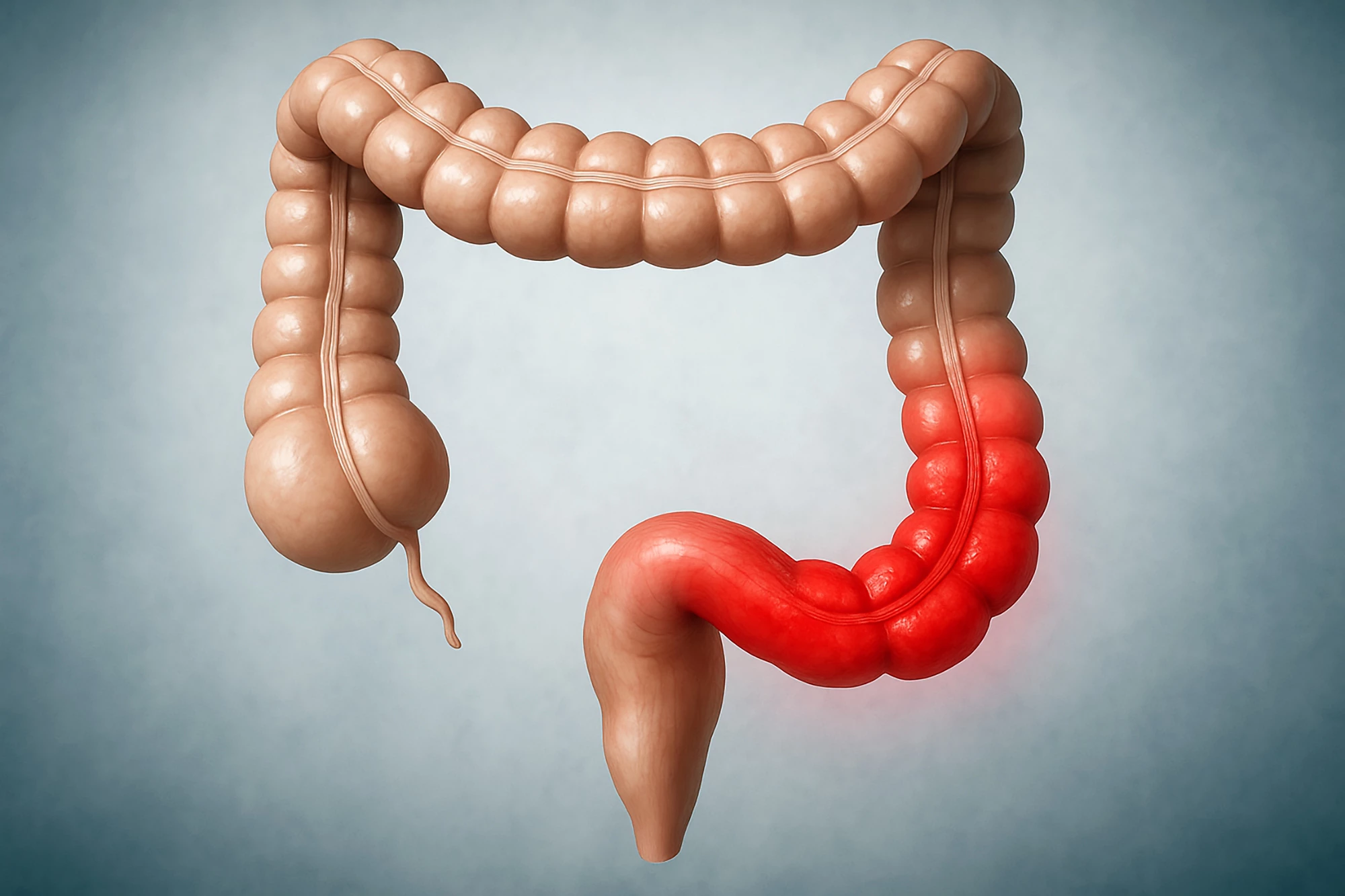
Affecting around five to 10 million people worldwide, ulcerative colitis (UC) is a long-term inflammatory disease that causes the gut lining to become swollen and develop ulcers. It leads to ongoing symptoms like frequent diarrhea, abdominal pain, fatigue, and an urgent need to use the bathroom. There’s no cure.
Current treatments aim to reduce inflammation, control symptoms, and prevent flare-ups. Now, a recent clinical trial of a new therapy, obefazimod, developed by French biotech company Abivax, has produced very promising results in the treatment of moderate-to-severe UC.
“Despite advances in care, many patients with ulcerative colitis continue to struggle with inadequate disease control and safety concerns that significantly impact their quality of life,” said Silvio Danese, MD, PhD, Research Committee Chair of United European Gastroenterology (UEG), Europe’s leading authority for digestive health. “The outstanding results shared today demonstrate meaningful improvements across a spectrum of patients with ulcerative colitis, ranging from those who were naïve to advanced therapies to those who have failed up to 4+ lines of prior advanced therapy, including JAK inhibitors.”
The results of the eight-week Phase 3 ABTECT trials were presented at the recent UEG congress 2025, held in Berlin. The studies enrolled more than 1,270 patients, nearly half of whom had seen little or no benefit from other advanced therapies such as biologics or JAK inhibitors. Biologics are derived from natural sources, such as human or animal genes and microorganisms, and are designed to block specific immune system proteins that cause inflammation. Janus kinase (JAK) inhibitors inhibit the JAK enzyme, which is crucial for signaling pathways that promote inflammation.
Obefazimod, on the other hand, is an oral drug designed to calm the immune response that drives UC. It works by increasing the body’s production of a small regulatory molecule called microRNA-124 (miR-124), which helps rebalance immune activity and reduce inflammation in the gut. By targeting this natural control pathway rather than blocking specific immune signals like many current treatments do, obefazimod aims to control disease activity without broadly suppressing the immune system, potentially leading to fewer side effects and more sustained symptom relief.
After eight weeks of treatment, patients taking 50 mg of obefazimod daily were significantly more likely to achieve both clinical remission (near-complete disappearance of symptoms) and clinical response (substantial improvement in symptoms) compared to placebo.
The benefit was consistent across all patient groups:
- Among those who had not previously used advanced therapies, response rates were 28% higher than placebo.
- In patients who had failed four or more prior therapies, the difference was 29%.
- In a particularly difficult-to-treat group – those whose disease had not responded to JAK inhibitors – the response rate was 35% higher than placebo.
The drug also produced significant improvements in gut healing, seen in both endoscopic and tissue-level measures. In patients with no prior advanced therapy exposure, the 25-mg and 50-mg doses were similarly effective.
Importantly, the treatment was well-tolerated across the study population, with no new safety concerns identified. The safety profile, combined with the broad efficacy results, positions obefazimod as a potential new standard of care for UC, which affects millions of people worldwide.
“Taken together with the clinically meaningful improvements across all efficacy endpoints and a continued favorable safety profile, these findings highlight obefazimod’s potential in becoming the standard of care for treatment a broad spectrum of patients with ulcerative colitis,” Danese said.
Abivax is now continuing its Phase 3 development program as it moves closer to seeking regulatory approval for obefazimod. If approved, the therapy could offer a convenient oral alternative to existing injectable treatments and provide new hope for patients who have exhausted other options.
Source: Abivax