Switching off a single enzyme in immune cells protected mice from obesity, type 2 diabetes, and fatty liver disease in a new study, offering a potential new treatment target for metabolic disorders.
The worldwide prevalence of obesity more than tripled in the nearly 50 years between 1975 and 2022, according to the World Obesity Federation. There have been countless studies on the negative effects of overweight or obesity on health. Now, a new study by researchers at Monash University, Melbourne, and Baylor College of Medicine, Texas, has identified an enzyme, CAMKK2, that, when “switched off” in immune cells, prevented diet-induced obesity, diabetes and fatty liver disease in mice.
“Obesity is linked to ongoing, low-level inflammation in key organs that control metabolism, like the liver, fat tissue and muscles, and this kind of inflammation plays a role in conditions such as insulin resistance and type 2 diabetes,” said John Scott, PhD, a senior research fellow from the Monash Institute of Pharmaceutical Sciences (MIPS) and one of the study’s corresponding authors. “It’s widely accepted that a big driver of this process is the buildup of macrophages in these organs because when the body is under stress, such as from a high-fat diet, inflammatory macrophages switch to a faster but less efficient way of making energy.”
Macrophages, immune cells that live in our tissues, are responsible for engulfing and digesting bacteria, dead and damaged cells, and harmful invaders like cancer cells. They can also present antigens to T cells and initiate inflammation by releasing signaling molecules called cytokines that activate other cells. The CAMKK2 (calcium/calmodulin-dependent protein kinase kinase 2) enzyme is a key regulator of energy metabolism throughout the body and coordinates these macrophage-led inflammatory responses.
In the present study, the researchers wanted to determine how the enzyme contributed to the regulation of inflammation and whole-body metabolism during diet-induced obesity. They genetically engineered mice so that they lacked CAMKK2 only in myeloid cells, the collective term for a group of immune cells that develop from bone marrow stem cells and includes macrophages, neutrophils, and dendritic cells. CAMKK2 knockout mice and normal control mice were fed a high-fat diet for several weeks. The scientists measured body weight, fat mass, energy expenditure, food intake, blood sugar and insulin responses, as well as fat and liver tissue (via microscopy), gene expression (via RNA sequencing), and macrophage metabolism.
They found that mice without CAMKK2 in macrophages resisted high-fat-diet-induced weight and fat gain. Although they didn’t eat less food, they burned more energy. These mice also had lower blood sugar and insulin levels, with much better glucose tolerance and insulin sensitivity. Additionally, their fat, liver, and muscle tissues handled sugar more effectively, and they were protected from fatty liver disease.
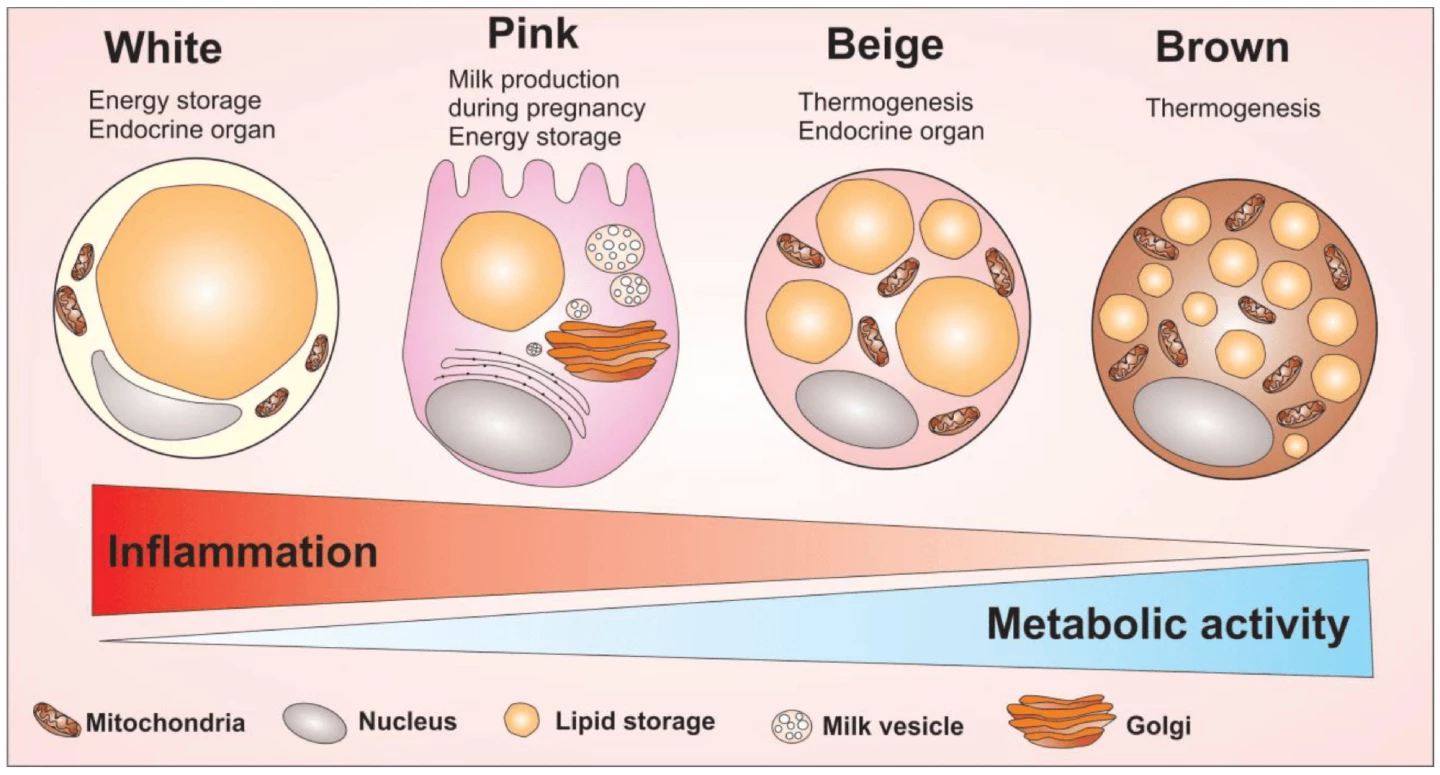
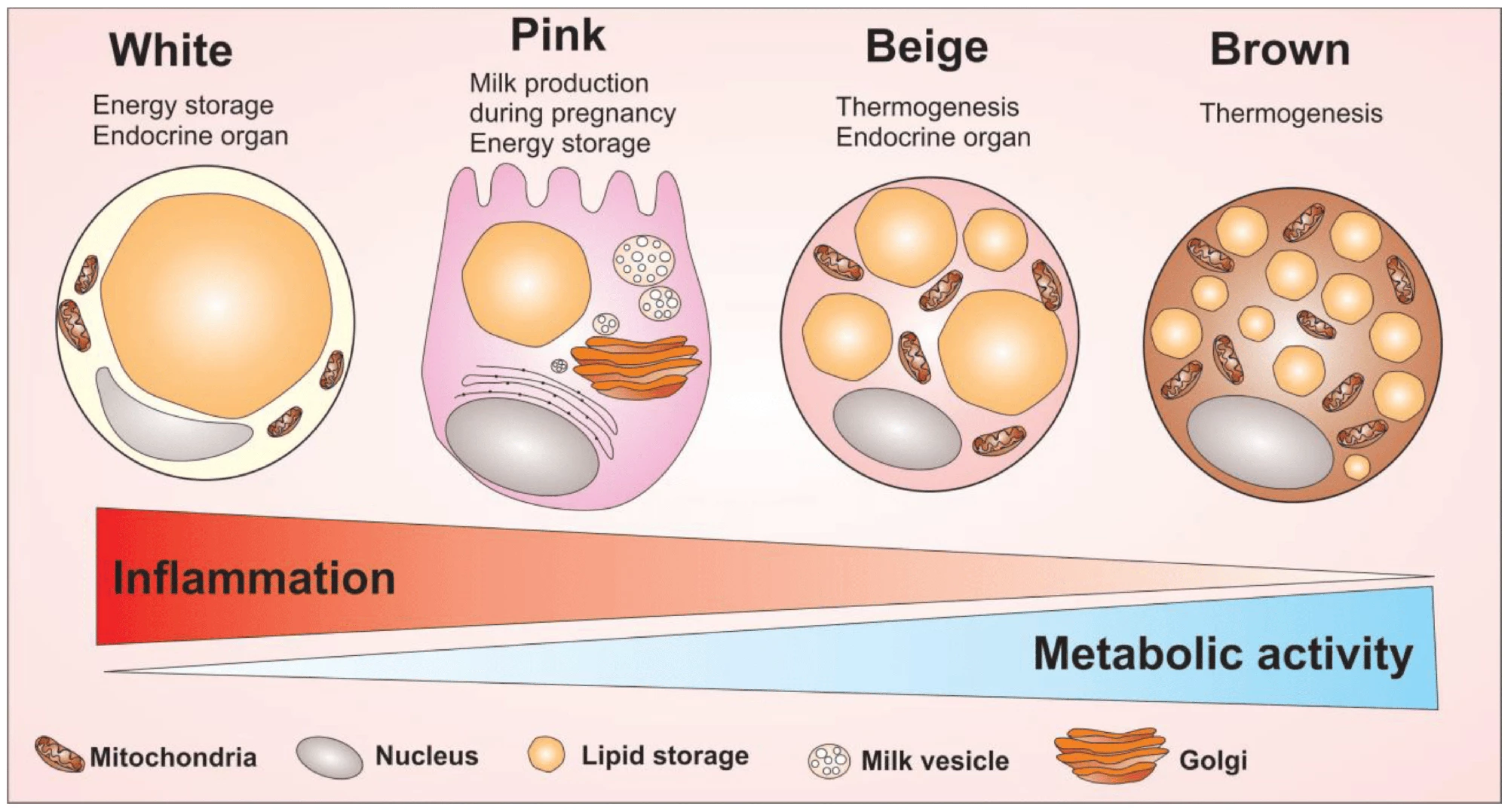
Normal obesity causes macrophages in fat tissue to shift toward a pro-inflammatory state, which drives insulin resistance. Without CAMKK2, macrophages had an anti-inflammatory profile, produced few inflammatory signals, and showed a preference for burning fat as fuel through increased fatty acid oxidation and improved mitochondrial function. This metabolic rewiring increased mitochondrial activity (energy production by the cells’ internal machinery) and energy efficiency. Adipose, or fat, tissue in the knockout mice was remodeled, too. It showed a “beiging” effect; gene programs that promoted fat burning and thermogenesis were upregulated.
“Our findings show that when the CAMKK2 gene is removed from certain immune cells (in this case, macrophages), fat tissue shifts its activity in a healthier direction,” Scott said. “The genes in the fat start working in ways that support better metabolism and reduce harmful inflammation.
“In short, we’ve identified that CAMKK2 has direct control of regulating immune cell and whole-body metabolism, making it a promising new therapeutic target for treating obesity and related metabolic disorders.”
The study’s main limitations include the fact the findings may not fully translate from mouse models to humans. Further, while macrophages showed more fat burning in dishes, it wasn’t directly proven that this happened to the same extent inside live animals. Also, a compound called STO-609 has been used to inhibit CAMKK2, but it is not selective enough and has poor drug-like properties, making it unsuitable for clinical use.
Putting aside these limitations, which can be addressed in future studies, the findings from the current study suggest that targeting CAMKK2 in macrophages could help prevent or reverse obesity-related insulin resistance, fatty liver, and metabolic disease. Additionally, since macrophage-driven inflammation also plays a role in atherosclerosis, infections, and some cancers, CAMKK2 inhibition could have broader benefits.
The study was published in the journal Molecular Metabolism.
Source: Monash University