Fraunhofer researchers have presented a magnesium-based "Powerpaste" that stores hydrogen energy at 10 times the density of a lithium battery, offering hydrogen fuel cell vehicles the ability to travel further than gasoline-powered ones, and refuel in minutes.
Typically, of course, hydrogen fuel cell vehicles carry their H2 fuel in gaseous form, stored in tanks at pressures around 700 bar (10,150 psi). These tanks are fairly large and heavy, which counteracts one of hydrogen's key advantages over today's lithium batteries – its higher energy density. The high pressures involved also make hydrogen an impractical option for powered two-wheelers like motorcycles and scooters.
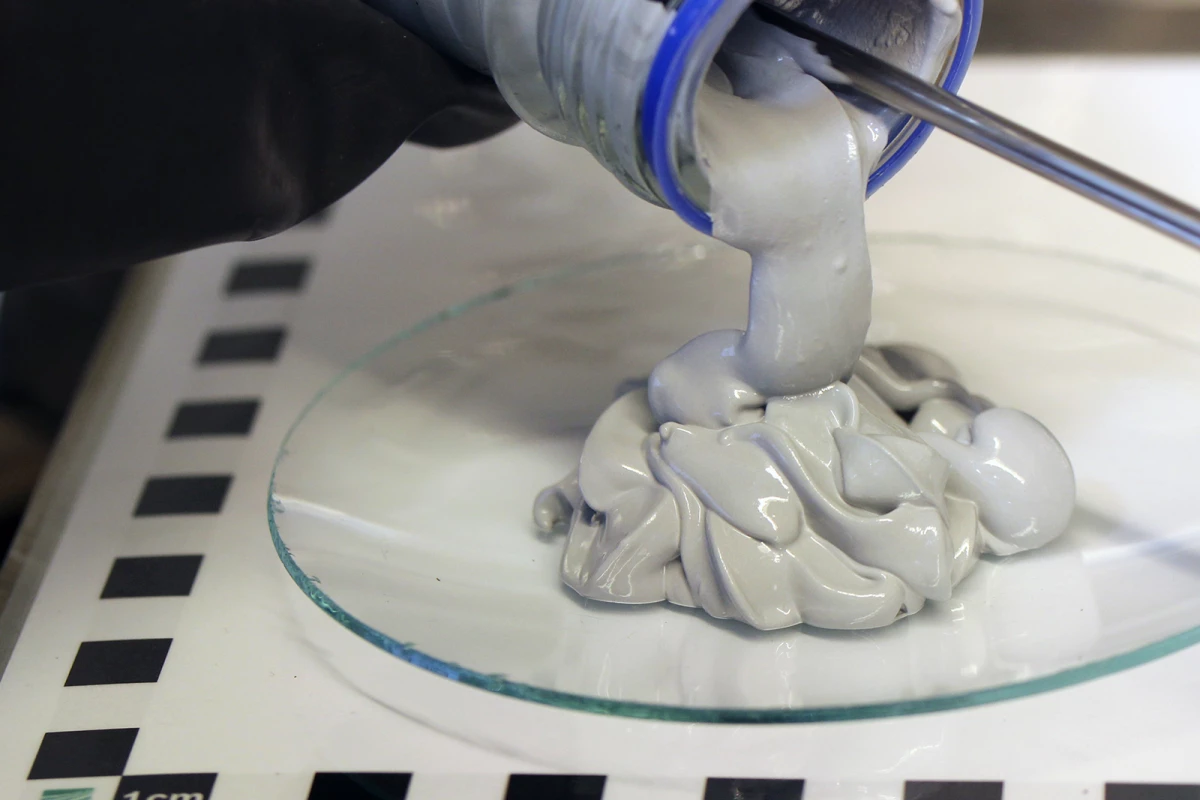
But a team based at the Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM in Dresden have come up with an interesting new way to store and carry hydrogen energy, in the form of a magnesium hydride-based "Powerpaste" that stores the hydrogen in a chemical form, at atmospheric pressure, ready for release when needed.
To produce the paste, magnesium is combined with hydrogen at around 350 °C (662 °F) and five to six times atmospheric pressure to form magnesium hydride. An ester and a metal salt are added to complete the process and form a viscous gray goop that can be loaded into cartridges.
In Powerpaste form, it's completely stable at temperatures up to 250 °C (482 °F). It carries 10 times the energy of a similar weight in lithium batteries, and substantially more than a 700-bar H2 tank of the same weight. The researchers say vehicles running on a Powerpaste powertrain can expect a range "comparable to – or even greater than – gasoline."
When it comes time to release the energy, a plunger mechanism extrudes the paste into a chamber where it reacts with water to release hydrogen at a dynamically controlled rate, which then feeds a fuel cell to create electrical power with which to run an EV powertrain or other device. Part of the paste's impressive energy density comes from the fact that half of the hydrogen released comes from the water it reacts with.

Refueling a Powerpaste scooter, for example, would be a matter of pulling out the cartridge and replacing it with a full one at a service station. In this way, this stuff could become something like a bottle of BBQ gas: an easily replaceable way to use clean energy in a range of devices. The Fraunhofer team speaks of using it in large drones, putting a multiplier on their flight time and range, or in portable electric appliances, such as a paste-powered camping toaster or kettle, for example.
And where removable cartridges might be just the ticket for some applications, others might find it easier just to pump the stuff into a tank – for example, fuel cell cars and trucks, aircraft and larger applications. The team says this sludgy goop can be supplied via standard filling lines with "relatively inexpensive equipment."
Logistically, this stuff seems much simpler than regular gaseous (and certainly liquid) H2. It can be trucked around in barrels or tankers, and left more or less anywhere without danger. Fraunhofer IFAM is building a Powerpaste production plant at its own facilities, which will open later this year and have the capacity to make up to four tons of paste a year for pilot programs and industry evaluation. It also has a power generator up and running on the test bench in the lab.
Questions remain, though. What happens to the magnesium as the paste is spent? Does it get recycled back into the process? Do the energy density figures quoted above take into account the entire system required, or just the fuel storage? How much water does it use for a given amount of energy, and how much water do you need to add when you fill up, given that fuel cells produce water as a by-product?
Perhaps most importantly, how energy-efficient is the process of making this paste? Let's not forget, one of the black marks against clean hydrogen in fuel cell systems is its horribly inefficient use of clean energy. Store clean energy in a battery and you'll get more than 90 percent of it back at the wheels. Store it in hydrogen and you torch at least half of it in lossy processes along the way.
The production of Powerpaste requires heat, pressure and industrial processes, all of which cost energy. Add to that the financial and energy costs of storage and transport, and the equations are likely to make gaseous H2 look like a very frugal little energy holder by comparison.
As long as Powerpaste is produced using nothing but clean energy, it still looks like a clean fuel – and perhaps a very convenient one that we can imagine rolling out to gas stations without too much fuss. But in a world where clean energy production is still limited, it may end up looking like a wasteful use of our solar and wind resources.
And then there's the matter of cost; how will Powerpaste compare to gasoline on a fill-up, given that it'll deliver roughly the same range? Certainly, it'll be a lot to begin with and would come down with scale and the price of clean H2. But the added processing and transport costs will make it more expensive than straight hydrogen gas, and a ballpark future figure could definitely be instructive. We'll put these questions to the researchers and see how things start to stack up.
Source: Fraunhofer