Not-so-fun fact: our oceans hold 150 times more carbon dioxide than the Earth's atmosphere. Adding to that causes ocean acidification, which can disrupt marine food chains and reduce biodiversity.
Addressing this could not only help restore balance to underwater ecosystems, but also take advantage of an opportunity to sustainably use this stored CO2 for a variety of purposes – including producing the industrial chemicals needed to make plastic.
The first towards this is called Direct Ocean Capture – which refers to removing dissolved carbon directly from seawater – happens through electrochemical processes. While there are a bunch of companies working on this, it hasn't extensively been applied at scale yet, and the cost benefit doesn't look great at the moment (it's estimated that removing 1 ton of CO2 from the ocean could cost at least US$373, according to Climate Interventions).
Scientists from the Chinese Academy of Sciences and the University of Electronic Science and Technology of China – both in Shenzhen, China – have devised a DOC method which involves converting the captured CO2 into biodegradable plastic precursors. This approach is also described as operating at 70% efficiency, while consuming a relatively small amount of energy (3 kWh per kg of CO2), and working out to an impressive $230 per ton of CO2.
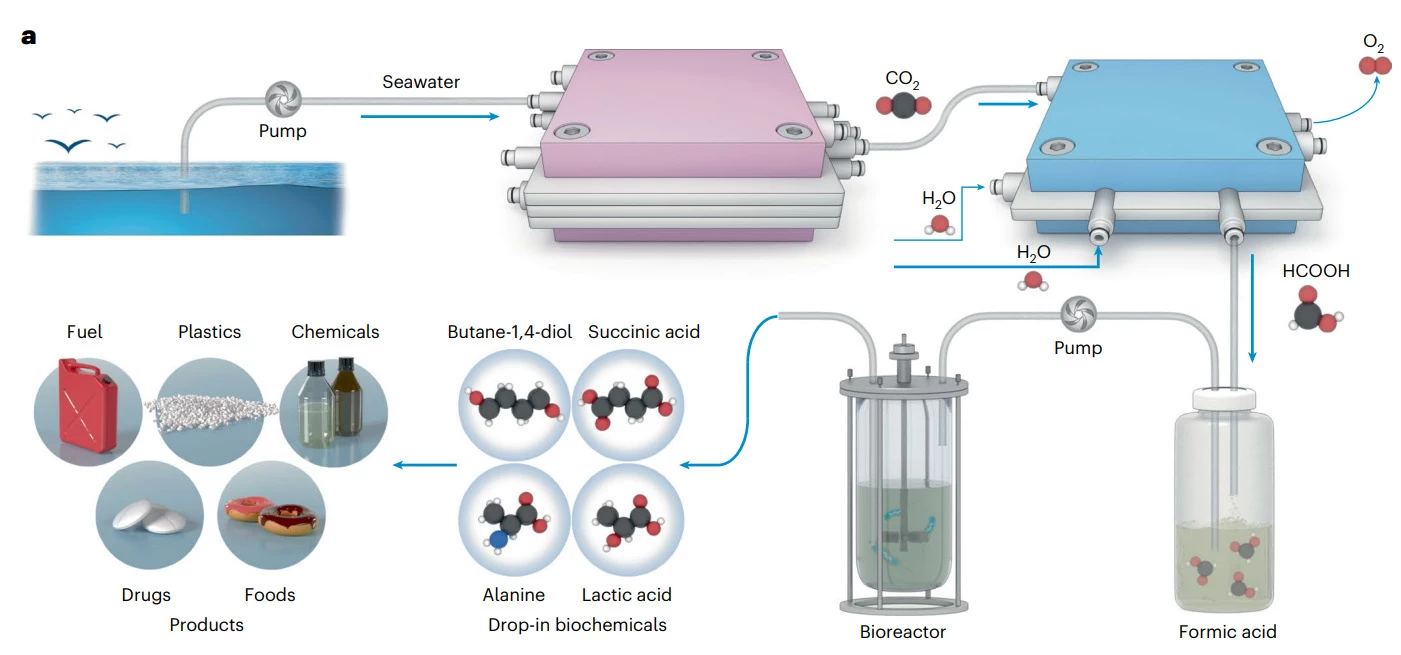
What's also worth noting is the use of modified marine bacteria for the last step. Here's a breakdown of the process, described in a paper appearing in Nature Catalysis:
First, electricity is used in a special reactor to acidify natural seawater. This converts the invisible, dissolved carbon into pure gas, which is collected. The system then restores the water's natural chemistry before returning it to the ocean.
Next, the captured CO2 gas is fed into a second reactor containing a bismuth-based catalyst to yield a concentrated, pure liquid called formic acid. Formic acid is a critical intermediate because it is an energy-rich food source for microbes.
Engineered marine microbes, specifically Vibrio natriegens, are fed the pure formic acid as their sole source of carbon. The microbes metabolize the formic acid and efficiently produce succinic acid, which is then used directly as the essential precursor to synthesize biodegradable plastics, such as polybutylene succinate (PBS).

That's a pretty good start. The researchers note there's room for optimization to boost yields and integrate this system into industrial processes. It could also be altered to produce chemicals for use in fuels, drugs, and foods.
It also remains to be seen how quickly the team can commercialize this DOC method, because it may have formidable competition. For example, Netherlands-based Brineworks says it will get to under $200/ton by 2030 with its electrolysis-based solution. The next couple of years will be worth watching in this fascinating niche of decarbonization.
Sources: Chinese Academy of Sciences, University of Electronic Science and Technology of China via Scimex




