The first results from Phase 3 trials testing MDMA-assisted psychotherapy for post-traumatic stress disorder (PTSD) have been announced. The extremely promising data are the first Phase 3 results to be revealed for any psychedelic-based therapy, paving the way for a landmark US Food and Drug Administration (FDA) approval in 2023.
For decades the Multidisciplinary Association of Psychedelic Studies (MAPS) has been working on establishing MDMA-assisted psychotherapy as a novel treatment for severe PTSD. Out of all the research going on in the psychedelic renaissance, from psilocybin for depression to LSD for pain, these MDMA studies have always been at the front of the pack.
Now, MAPS has announced the first Phase 3 data for any psychedelic-assisted therapy research to date. The results have yet to be published in a peer-reviewed journal, but are scheduled to soon appear in Nature Medicine.
This first Phase 3 trial included 90 patients with severe, chronic PTSD, from a variety of different causes (abuse, combat, sexual trauma). All patients completed a 12-week treatment program composed of three full day sessions with either MDMA or placebo, plus weekly non-drug therapy sessions.
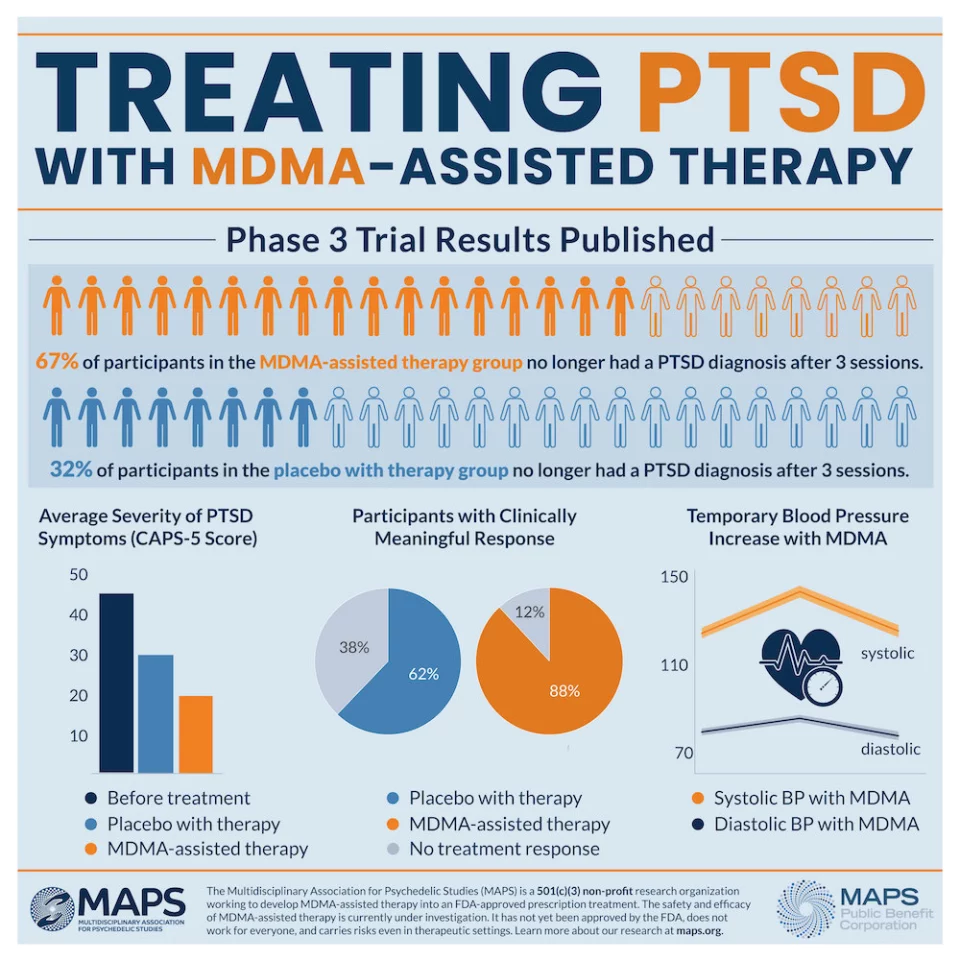
As with prior MDMA studies, no serious adverse effects were detected beyond transient, mild symptoms during drug treatment such as nausea or sweating. No increases in suicide risk, or potential for abuse, were noted in the MDMA group relative to placebo.
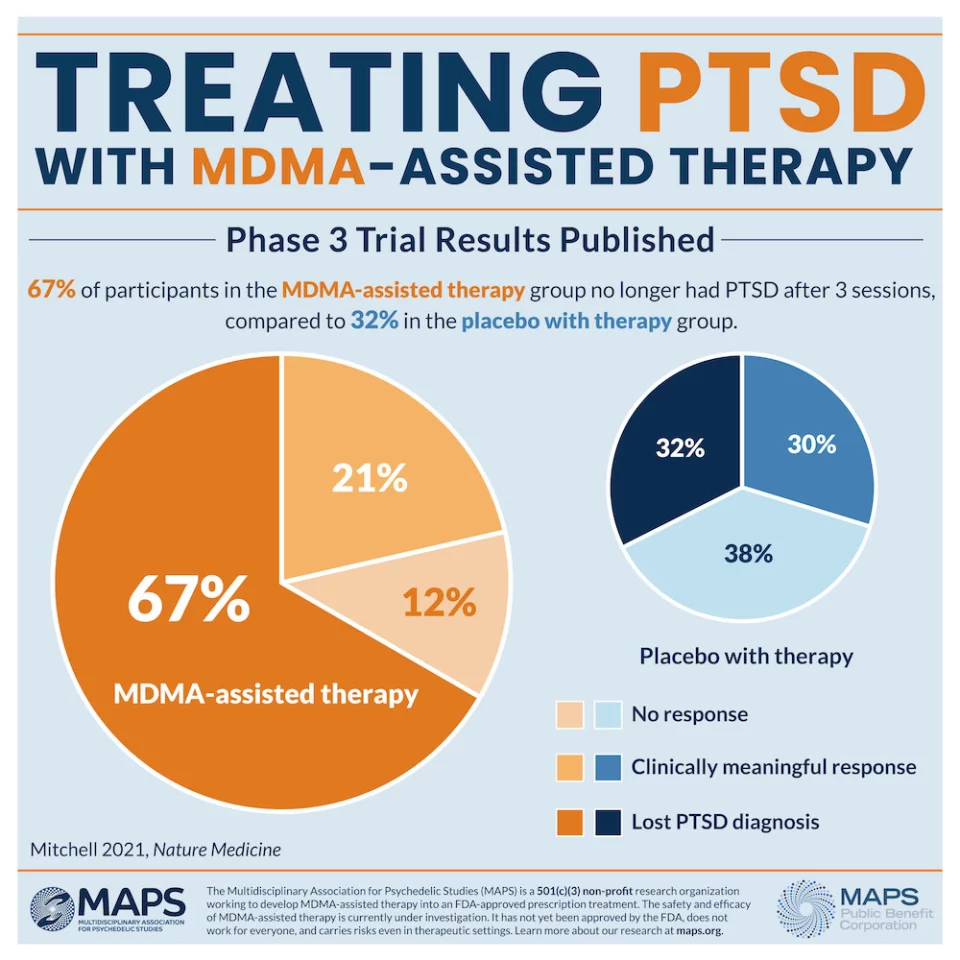
Two months after the treatment program the subjects were independently assessed for symptom severity and 67 percent of the MDMA cohort no longer qualified for PTSD diagnosis. This compares to 32 percent in the placebo group no longer qualifying for PTSD. Overall, a striking 88 percent of those in the MDMA group experienced clinically significantly reductions to symptoms.
It is important to note this was not a treatment naive cohort of PTSD patients. Instead, the patients recruited had all suffered with the condition for an average of 14 years and displayed resistance to current treatment methods.
“As a result of this study and through the persistent and consistent application of scientific rigor, we have demonstrated that MDMA-assisted therapy is likely to provide relief for people diagnosed with PTSD,” says Rick Doblin, Executive Director of MAPS. “Far from having no medical benefit MDMA, when combined with talk-therapy in this protocol, has the potential to catalyze the therapeutic process and generate positive mental health outcomes.”
These new results are effective replications of prior Phase 2 trials, which previously led the FDA to assign MDMA-assisted psychotherapy for PTSD a Breakthrough Therapy designation. The research has also been granted Expanded Access by the FDA, allowing patients access to the therapy prior to full market approval. MAPS has revealed 50 patients will access the treatment through this program.
MAPS has previously looked to 2021 or 2022 for FDA market approval, however, the COVID-19 pandemic has slowed progress down. A second Phase 3 trial, necessary for FDA approval, is ongoing and MAPS now is aiming for 2023 as the year the first psychedelic medicine will reach public clinics.
MDMA-assisted psychotherapy is also currently under investigation as treatment for alcoholism, eating disorders, end of life anxiety in terminal cancer patients, social anxiety in autistic adults, and as an adjunct to couples therapy.
Source: MAPS






