A 67-year-old London man with lung cancer has been the first to receive a new cancer vaccine as part of an international trial. The early-stage research will test the immune therapy’s safety and whether it can be used together with existing cancer treatments.
When you hear ‘vaccine,’ you probably think of the jab for the flu or COVID-19. However, a vaccine is any substance that helps the body’s immune system recognize and fight diseases, including cancer.
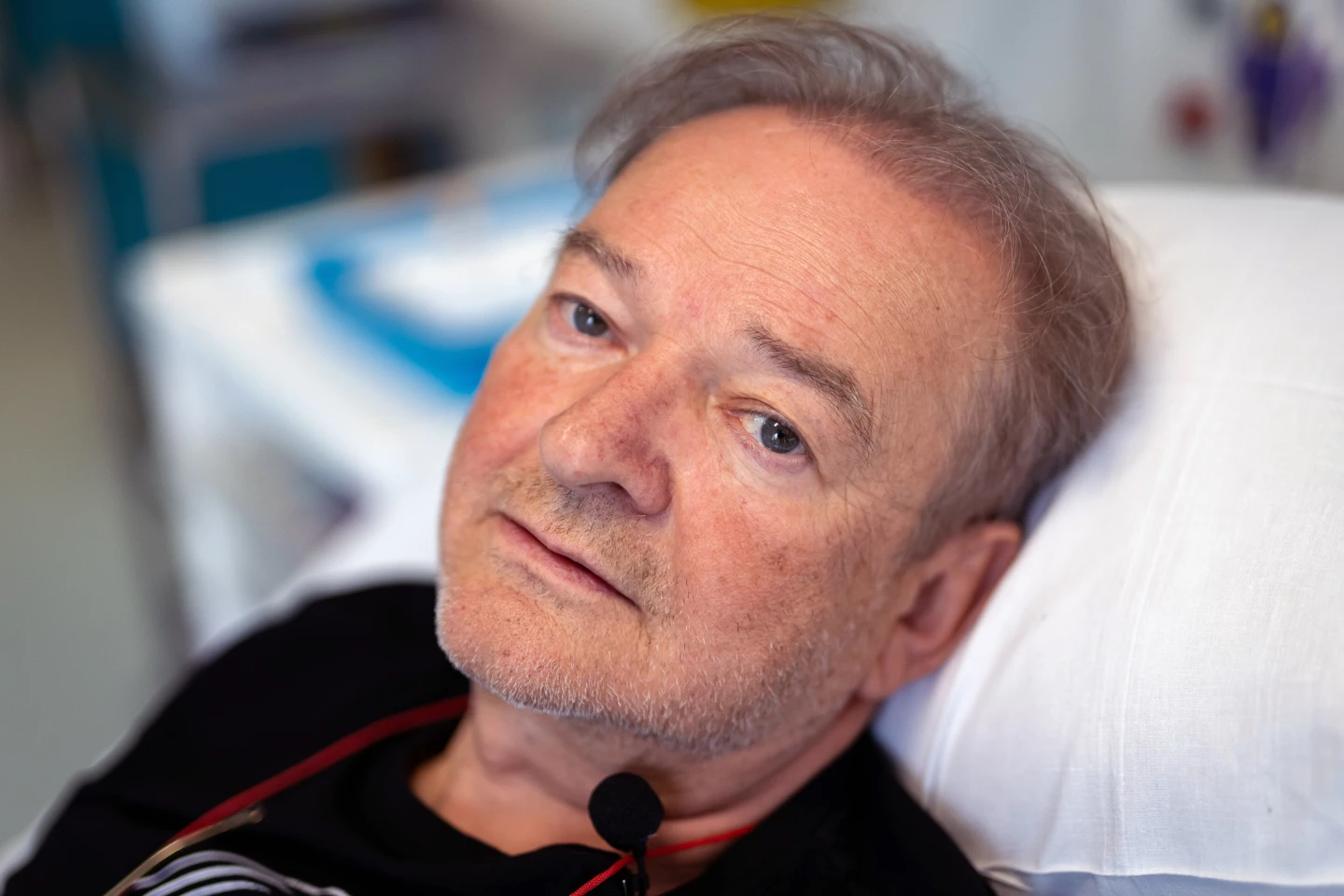
A 67-year-old lung cancer patient from London has been the first recipient of a new investigational cancer vaccine at the National Health Service (NHS) University College London Hospitals (UCLH).
“Lung cancer remains the leading cause of cancer deaths worldwide, with an estimated 1.8 million deaths in 2020,” said Siow Ming Lee, professor of medical oncology at University Hospital London and leader of the UK arm of the study. “We are now entering this very exciting era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer … We hope this will provide an opportunity to further improve outcomes for our NSCLC [non-small cell lung cancer] patients, whether in the early or advanced stages.”
Non-small cell lung cancer, or NSCLC, is one of two primary kinds of lung cancer and is the most common kind. The other kind is small cell lung cancer (SCLC). In NSCLC, cancer cells originate in the lung tissue, and although it grows slower than the small cell variant, NSCLC has often spread to other body parts by the time it’s diagnosed.
The novel vaccine, BNT116, made by the German biotech company BioNTech, uses messenger RNA (mRNA) to present markers from the tumor to the patient’s immune system so it can recognize and fight the cancer cells carrying the tumor markers. The vaccine works selectively on cancer cells via the patient’s immune system, rather than, say, chemotherapy, which can be toxic to both cancerous and healthy cells.
“The strength of the approach we are taking is that the treatment is aimed at being highly targeted towards cancer cells,” said Dr Sarah Benafif, who’s leading the trial.
About 130 participants will be enrolled in the study across seven countries. Patients at different stages of NSCLC, from those in the early stage before treatment with surgery or radiation therapy to those with late-stage or recurrent cancer. This early-stage research will determine whether BNT116 is safe and well-tolerated as a standalone anti-tumor treatment and whether it works synergistically when it’s given alongside established NSCLC treatments.
Janusz Racz is the trial’s first participant. As someone who works in a scientific field, he is glad to be able to contribute to the advancement of cancer treatment.
“I thought it over, and … decided to take part because I hope it will provide a defense against cancer cells,” Racz said. “But I also thought that my participation in this research could help other people in future and help this therapy become more widely available.
“As a scientist myself, I know that science can only advance if people agree to participate in programs like this,” continued Racz.”I work in artificial intelligence, and I am open to trying new things. My family did research about the trial, too, and they supported me taking part.”
Source: UCLH/NHS






