Although we've seen a number of systems that use sunlight to purify tainted water, their output is often quite limited. A new loofah-inspired hydrogel, however, uses sunlight to treat much more water in one go … enough to meet a person's daily needs.
Existing solar-still-type systems use sunlight to heat dirty water to the point that it starts evaporating. The resulting pollutant-free water vapor condenses on a surface within the still, then trickles down into a collection reservoir. It's a clever setup, but it only produces potable water as long as the sun is shining – even then, it's a slow process.
Materials known as temperature-responsive hydrogels offer an alternative, as they soak up dirty water at cool temperatures, then expel it (in purified form) when heated. According to Rodney Priestley, Xiaohui Xu and colleagues at Princeton University, however, the closed pores of most such gels don't allow water to be treated fast enough for most people's needs.
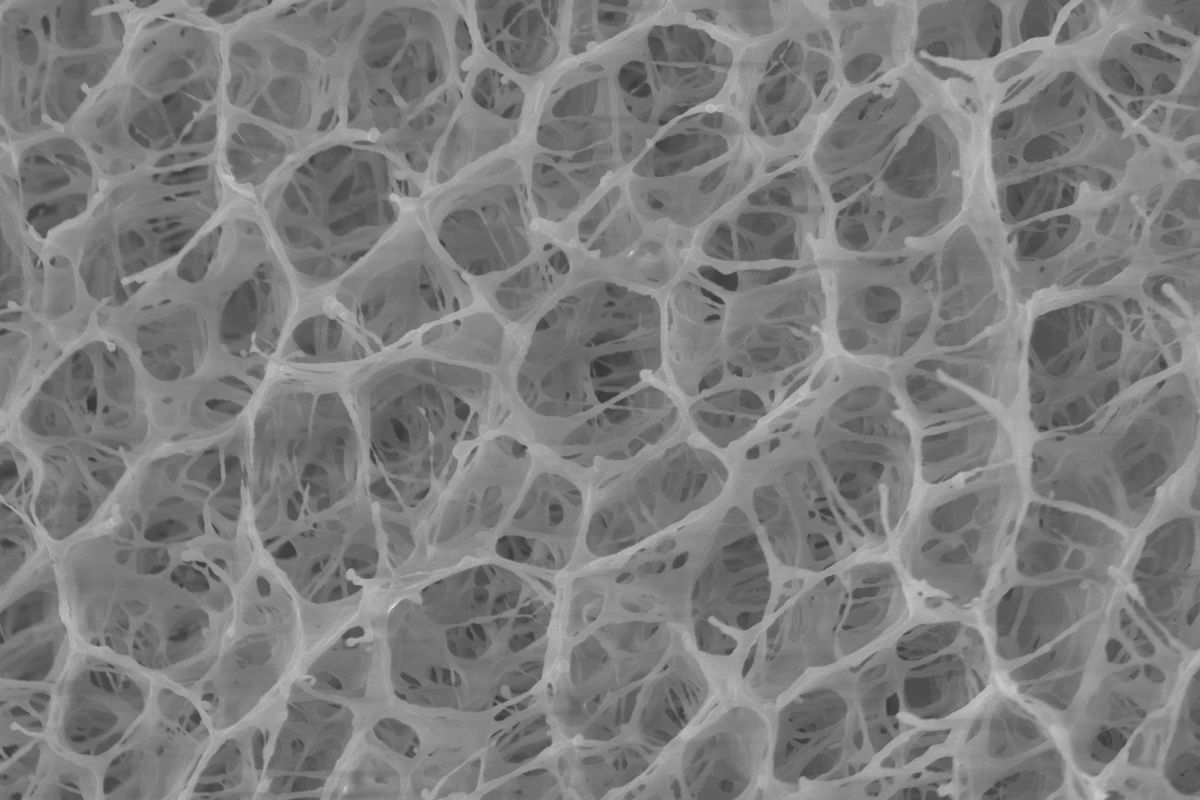
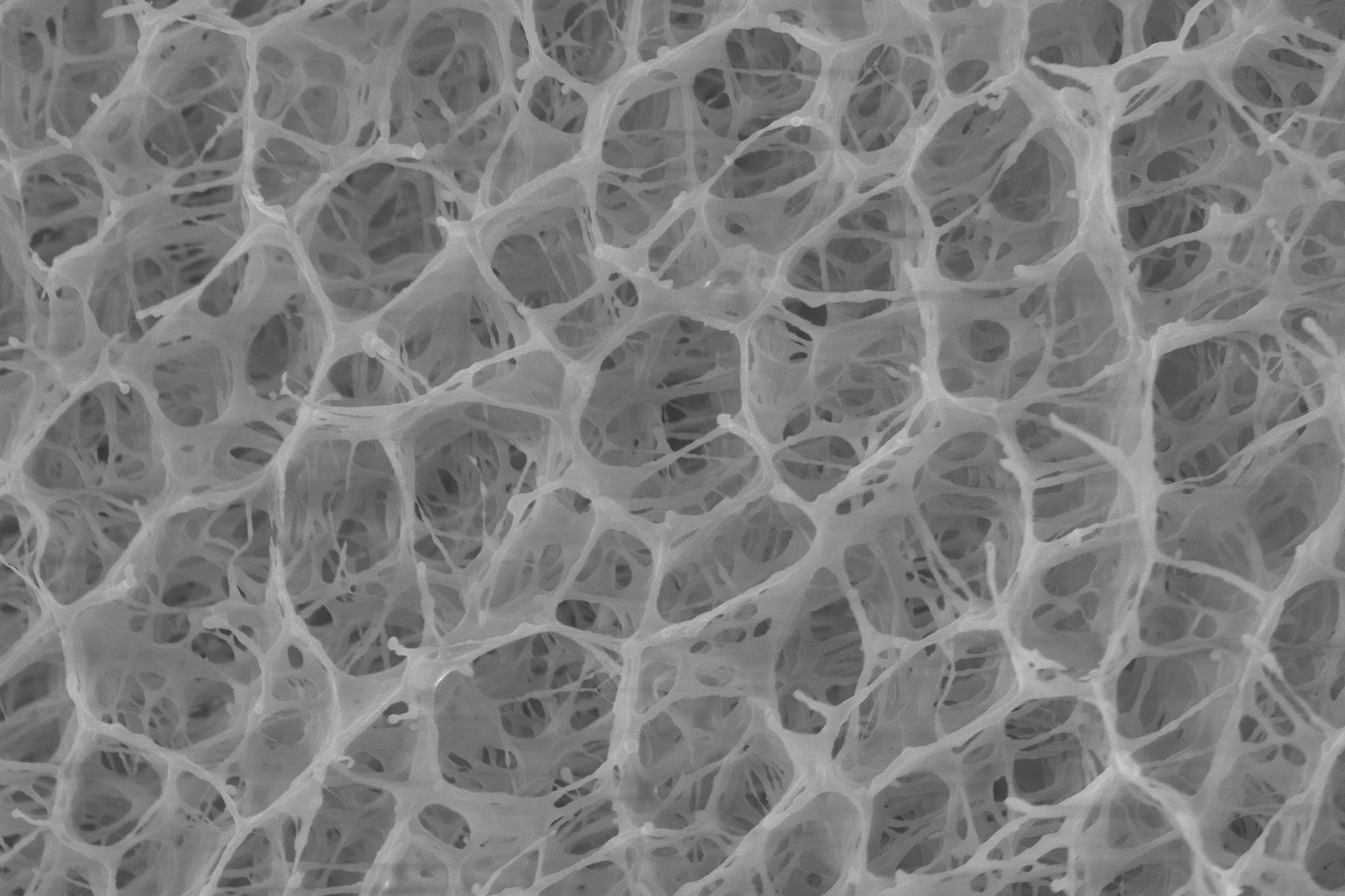
With that limitation in mind, the scientists set out to replicate the very open-pored design of the natural loofah sponge.
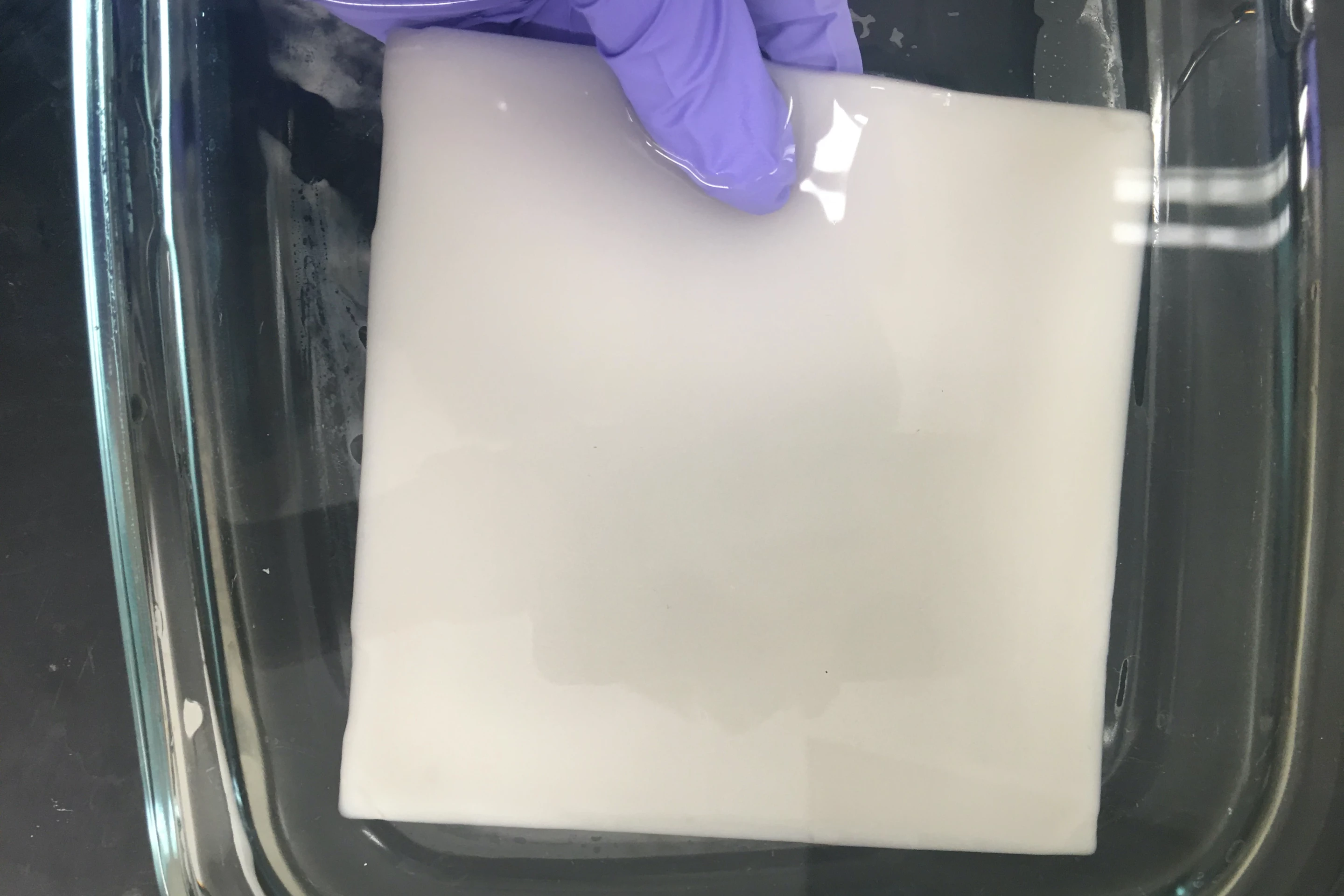
They started by using a water/glycol mixture as a unique polymerization medium, to produce a PNIPAm (poly(N-isopropyl acrylamide)) hydrogel with the large, open pores that they wanted. The scientists then coated the gel's inner pores with two other polymers – polydopamine (PDA) and poly(sulfobetaine methacrylate) (PSMBA).
The resulting material soaked up tainted water at room temperature, then released 70% of that water within 10 minutes when heated by simulated sunlight – this was four times faster than a previously tested closed-pore hydrogel. Additionally, even under lighting conditions that simulated partly cloudy skies, the loofah gel still released a similar amount of water within 15 to 20 minutes.

So, how does it actually work?
Xu tells us that the hydrogel is hydrophilic (water-attracting) at cool temperatures, but becomes hydrophobic (water-repelling) when heated. Pollutants such as organic dyes (which were used in lab tests) do get pulled in along with the water at cool temperatures, but because their molecules stick to the gel, they don't get expelled when the water is released at warmer temps. Droplets of oil pollution don't get drawn in in the first place, as the (then) hydrophilic gel rejects them.
In the lab tests, the loofah hydrogel was also successfully used to remove pollutants such as microplastics and heavy metals. Via two treatment cycles, the material was able to reduce chromium pollution from about 40 parts per million down to less than 0.07 parts per million, which is the allowable limit for drinking water.
And yes, Xu says that the loofah hydrogel can be reused simply by rinsing it with diluted acid or ethanol.
A paper on the research was recently published in the journal ACS Central Science.
Source: American Chemical Society