Using a popular culinary technique, researchers have created a foam infused with carbon monoxide to boost the effectiveness of autophagy inhibitors, an experimental cancer therapy that until now has produced mixed results in clinical trials. The novel foam offers a promising approach to improving treatments for different cancers.
Autophagy is a natural recycling process where cells degrade and recycle damaged or dysfunctional intracellular components to stop them from accumulating. A number of conditions, such as nutrient deprivation, oxidative stress and cancer cell proliferation, can initiate the degradation pathway. In cancer cells, autophagy is increased, suggesting that inhibiting it may be an effective way of treating the disease.
Drugs that inhibit autophagy, such as the antimalarials chloroquine and hydroxychloroquine, have been used as an experimental combination cancer treatment to boost the effectiveness of chemotherapies. However, results from clinical trials have produced inconclusive results, prompting researchers from the University of Iowa Carver College of Medicine to ask why.
“Within those clinical trials, they found mixed results; there was some benefit, but for many patients, there was no benefit, which really pushed researchers back to the drawing board,” said James Byrne, corresponding author of the study.
In looking for a reason why autophagy inhibition only worked some of the time, the researchers made a surprising discovery: in two of the trials, patients who actively smoked seemed to do better than non-smokers.
“When we looked at how the smokers did in those trials, we saw an increase in overall response in smokers that received the autophagy inhibitors, compared to (non-smoker) patients, and we also saw a pretty robust decrease in the target lesion size,” Byrne said.
Previous studies had found that autophagy is responsive to biological signaling molecules called gasotransmitters, a class of neurotransmitters that includes carbon monoxide (CO), a known component of cigarette smoke. So, the researchers focused on developing a means of delivering exogenous CO.
“We know that smokers have higher carbon monoxide levels and while we definitely don’t recommend smoking, this suggested that elevated carbon monoxide might improve the effectiveness of autophagy inhibitors,” Byrne said. “We want to be able to harness that benefit and take it into a therapeutic platform.”
Helpfully, Byrne specializes in creating gas-entrapping materials (GeMs), foams, gels and solids made from safe, edible substances that can be infused with different gas molecules. If you’ve watched one of the myriad cooking competition shows available to stream, you’ll have probably encountered molecular gastronomy and the creation of fancy culinary foams. The researchers used the same technique – a whipping siphon – here to create a drinkable foam infused with CO, which they called a CO-GeM.
First, in the lab, they tested the synergistic effect of CO with autophagy inhibitors in human prostate, pancreatic and lung cancer cells. Cells exposed to increasing doses of bafilomycin A1 (BAF-A1), chloroquine (CQ), and Lys05 demonstrated that the cytotoxicity (cell-damaging ability) of the inhibitors was increased in the presence of 250 ppm of CO. Importantly, CO did not affect cell viability in the absence of the autophagy inhibitor in cancer cells and normal human intestinal cells.
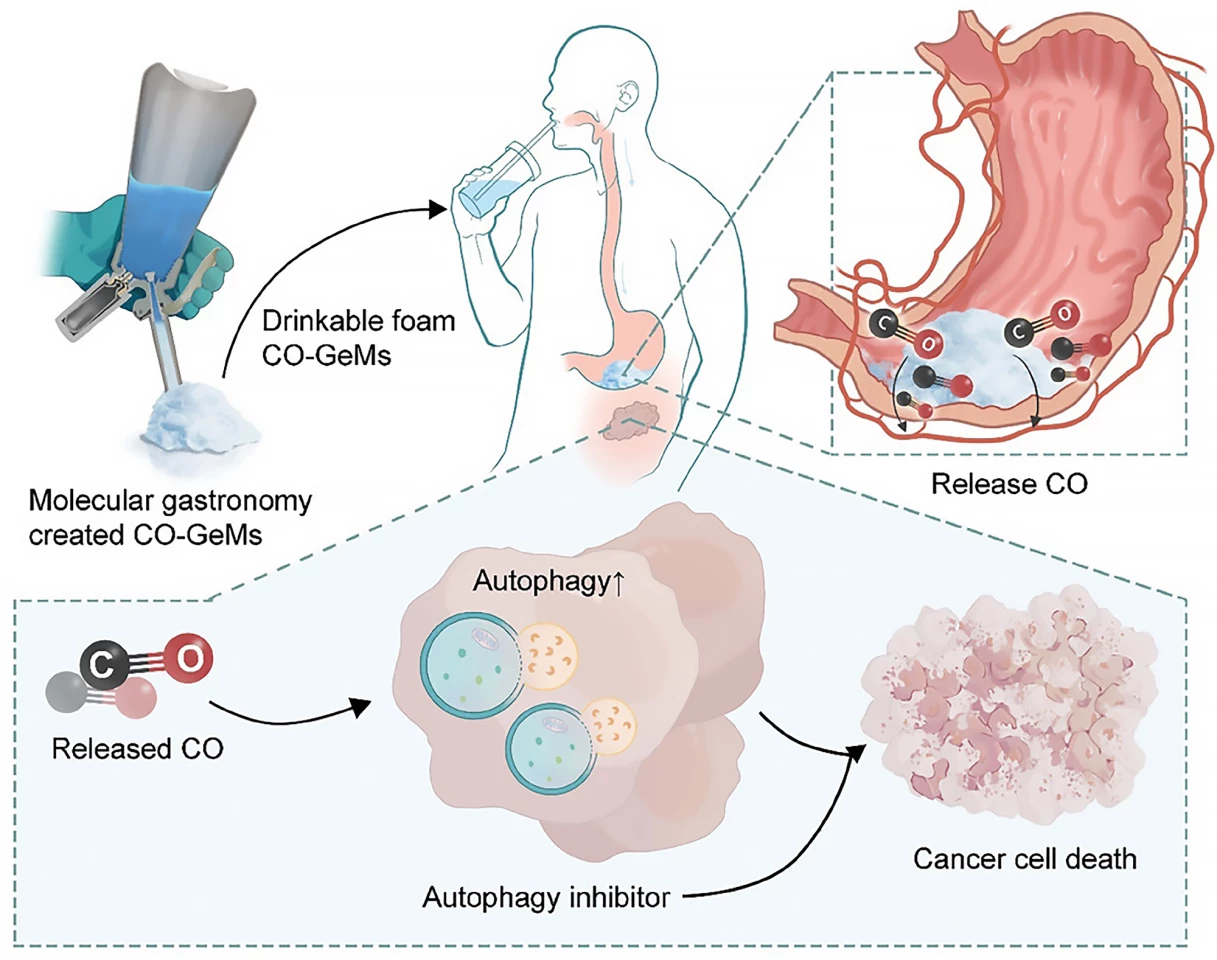
Next, they tested their CO-GeM foam in mouse models of prostate and pancreatic cancer, dividing the animals into four treatment groups: CO-GeM plus hydroxychloroquine (HCQ), CO-GeM alone, HCQ alone, and no treatment. By 21 days, a significant reduction in tumor growth was observed in mice receiving CO-GeM plus HCQ. The weights of the mice were stable throughout treatment, and they showed no signs of liver damage, suggesting the combination treatment was safe, biocompatible, and well-tolerated.
“The results from this study support the idea that safe, therapeutic levels of CO, which we can deliver using GeMs, can increase the anti-cancer activity of autophagy inhibitors, opening a promising new approach that might improve therapies for many different cancers,” said Byrne.
The translatability of the researchers’ findings to cancer therapies will depend on methods for CO delivery and the safety of the materials. Previous studies using inhaled CO have concluded that CO treatment is extremely safe, especially in immunocompromised patients. However, the researchers say physicians may more readily accept CO treatment if their novel foams are used instead of inhalation.
The researchers plan to work on dosing formulations of CO-GeMs to identify doses that lead to carboxyhemoglobin (COHb) levels – the standard method for confirming the degree of CO exposure – that fall under the FDA limit of 14% before testing the use of the novel foam combined with autophagy inhibitors in human clinical trials.
The study was published in the journal Advanced Science.
Source: University of Iowa Health Care






