Two innovative new developments out of the same laboratory have demonstrated that degraded cartilage can be repaired and regrown, first by using "dancing molecules" to target the proteins needed for tissue regeneration, then with the aid of a hybrid biomaterial that acts as scaffolding to encourage cartilage growth. They have the potential to do what nature can't do on its own – regrow cartilage – which could be used to ease all kinds of joint pain, including osteoarthritis, as well as do away with major surgeries such as total knee reconstructions.
“Cartilage is a critical component in our joints,” said lead researcher Samuel Stupp, from Northwestern University. “When cartilage becomes damaged or breaks down over time, it can have a great impact on people’s overall health and mobility. The problem is that, in adult humans, cartilage does not have an inherent ability to heal. Our new therapy can induce repair in a tissue that does not naturally regenerate. We think our treatment could help address a serious, unmet clinical need.”
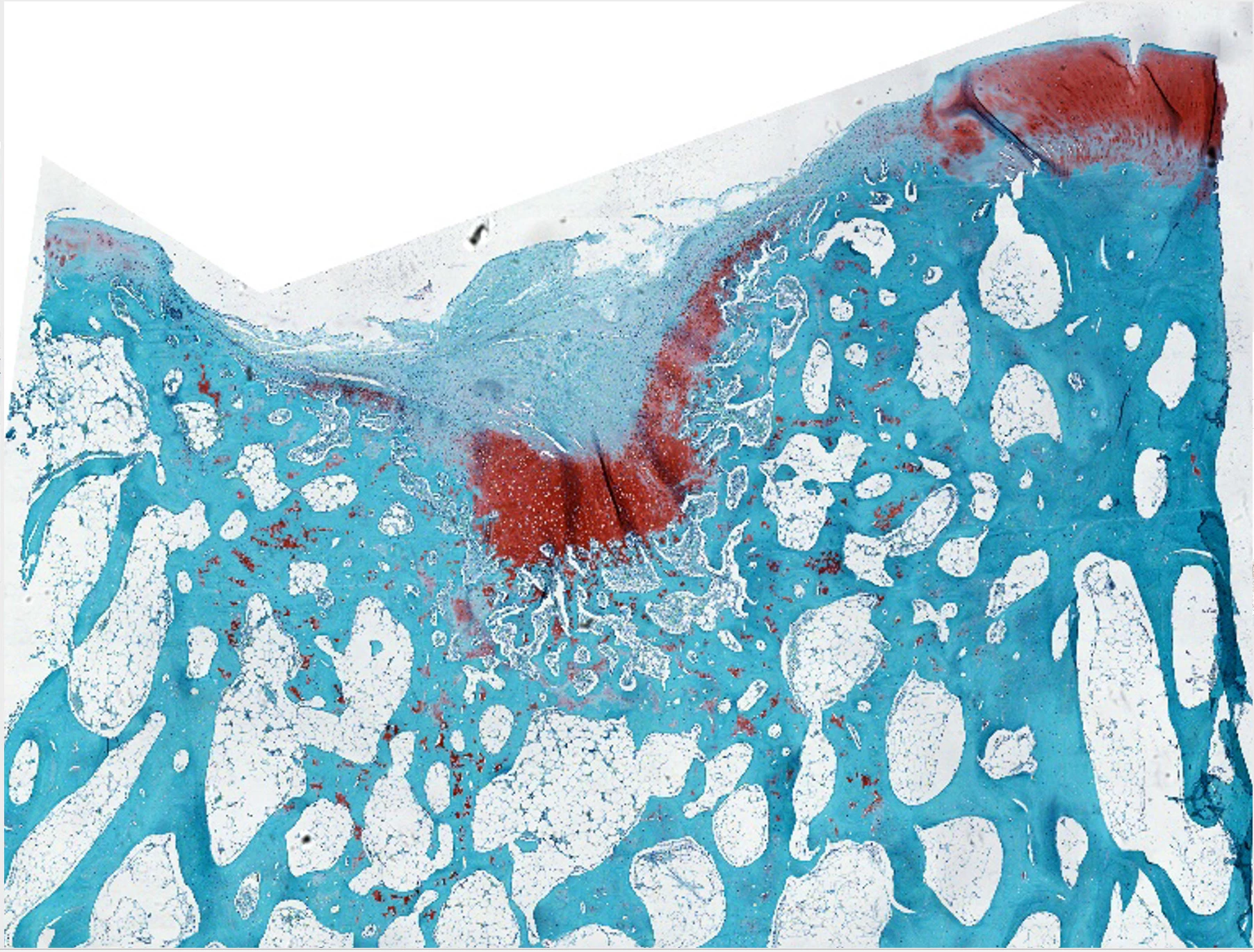
The hybrid biomaterial trial, which was used to regrow damaged cartilage in sheep joints, followed hot on the heels of the study using "dancing molecules," or synthetic nanofibers that contain hundreds of thousands of molecules that possess strong signals for cells. Altering their chemical structure to "dance" or move around quickly, Stupp and team found that these molecules were able to quickly locate and engage with cellular receptors. Inside the body, the nanofibers align with the extracellular matrix of surrounding tissue and mimic natural cellular communication.
“Cellular receptors constantly move around,” Stupp said. “By making our molecules move, ‘dance’ or even leap temporarily out of these structures, known as supramolecular polymers, they are able to connect more effectively with receptors.”
The team developed a circular peptide to then target the transforming growth factor beta-1 (TGFb-1) protein, which is found throughout the body and crucial in cartilage (and bone) growth. When comparing slow-moving molecules to the "dancing" assembly, the researchers found that the latter was significantly more effective at activating TGFb-1 receptors.

“After three days, the human cells exposed to the long assemblies of more mobile molecules produced greater amounts of the protein components necessary for cartilage regeneration,” Stupp said. “For the production of one of the components in cartilage matrix, known as collagen II, the dancing molecules containing the cyclic peptide that activates the TGF-beta1 receptor were even more effective than the natural protein that has this function in biological systems.”
The team is now testing this dancing molecules system on regenerating bone, with results to be published later this year. The team also hopes to take this development to clinical trial for spinal cord repair.
However, it's not the only discovery out of the Stupp lab, with another approach that saw cartilage regenerated in the joints of sheep. In the second study, instead of dancing molecules, the team developed a hybrid biomaterial made up of a bioactive peptide that binds to that all-important TGFb-1 protein and a modified hyaluronic acid, the natural goo-like substance that lubricates components of the body, including joints.
“Many people are familiar with hyaluronic acid because it’s a popular ingredient in skincare products,” Stupp said. “It’s also naturally found in many tissues throughout the human body, including the joints and brain. We chose it because it resembles the natural polymers found in cartilage.”
The team used this biomaterial to stimulate nanoscale fiber organization into bundles – mimicking the natural makeup of cartilage. This essentially formed a bio-friendly scaffolding that would encourage the body's cells to regenerate cartilage tissue on it.
This 'rubbery goo' biomaterial was inserted into the damaged knee cartilage of sheep, and within six months the tissue had shown enhanced repair as well as the growth of new cartilage made up of natural biopolymers (collagen II and proteoglycans). It resulted in pain-free movement and effective stability in the previously damaged joint, with the new cartilage structure remaining sound as the artificial scaffolding naturally degraded.


“A study on a sheep model is more predictive of how the treatment will work in humans,” Stupp said. “In other smaller animals, cartilage regeneration occurs much more readily.”
The team believes this thick paste-like biomaterial could be used in surgery as a less invasive way to encourage cartilage repair, compared to the current method of microfracture procedures.
“The main issue with the microfracture approach is that it often results in the formation of fibrocartilage – the same cartilage in our ears – as opposed to hyaline cartilage, which is the one we need to have functional joints,” Stupp said. “By regenerating hyaline cartilage, our approach should be more resistant to wear and tear, fixing the problem of poor mobility and joint pain for the long term while also avoiding the need for joint reconstruction with large pieces of hardware.”
The initial study was published in The Journal of the American Chemical Society, while the biomaterial study was published in the journal Proceedings of the National Academy of Sciences.