There’s a global shortage of blood supplies needed for life-saving transfusions due to factors that include an aging population with a higher demand for it and a lack of volunteer donors. However, even if there was an ample blood supply, it’s not as simple as just giving blood when it’s needed.
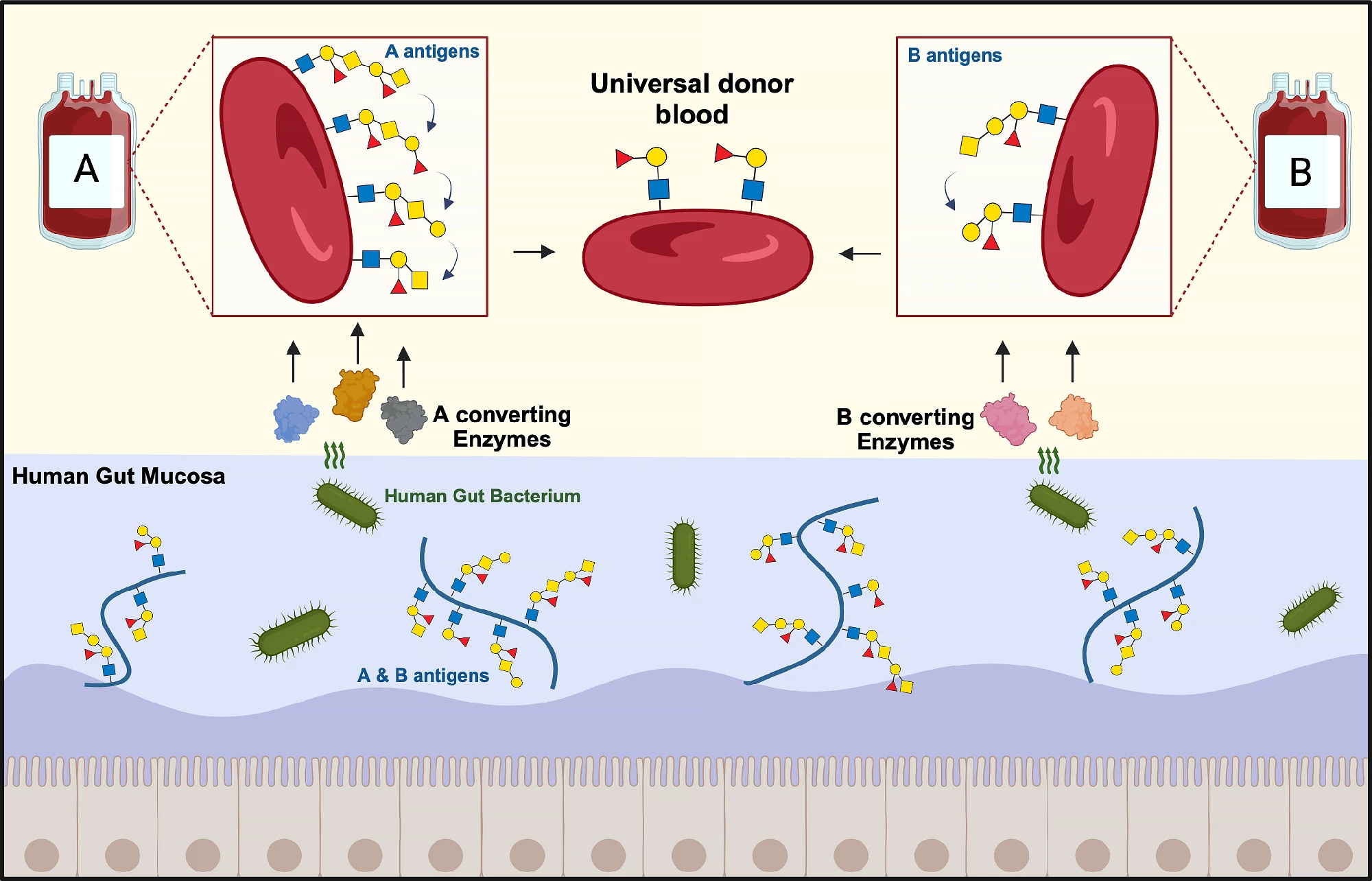
Each blood group (A, B, or AB) or type is identified by the presence of A and B antigens attached to sugar (oligosaccharide) chains on the surface of red blood cells. Blood cells in group O carry no antigens. When a blood transfusion is given, the donor and recipient's blood groups need to match. Otherwise, the immune system will attack and destroy the donated blood cells, causing a potentially fatal reaction.
Researchers at the Technical University of Denmark (DTU) and Lund University, Sweden, have used enzymes produced by a common gut bacteria to remove the A and B antigens from red blood cells, bringing them one step closer to creating universal donor blood.
“For the first time, the new enzyme cocktails not only remove the well-described A and B antigens, but also extended variants previously not recognized as problematic for transfusion safety,” said Maher Abou Hachem, co-corresponding author of the study and scientist at DTU’s Department of Biotechnology and Biomedicine.
As mentioned, the term ‘blood group’ denotes the combination of antigens present on the surface of a person’s red blood cells. By ‘extended variants,’ Abou Hachem is referring to blood group antigens discovered since the canonical four were more than 120 years ago. The International Society of Blood Transfusion (ISBT), defines a blood group system as a genetically discrete system of one or more antigens. As of November 2023, there were 45 recognized blood group systems containing 362 red blood cell antigens that are genetically determined by 50 genes.
The bacterium the researchers studied was Akkermansia muciniphila, a common resident of the healthy human gut lining. As its name, muciniphila, suggests, the bug loves mucins, the major component of the mucus that the lining produces. It uses enzymes to break down the mucins to create a source of carbon, nitrogen, and energy. Coincidentally, in addition to appearing on red blood cells, blood group or ABO antigens are also present in this mucosal lining.
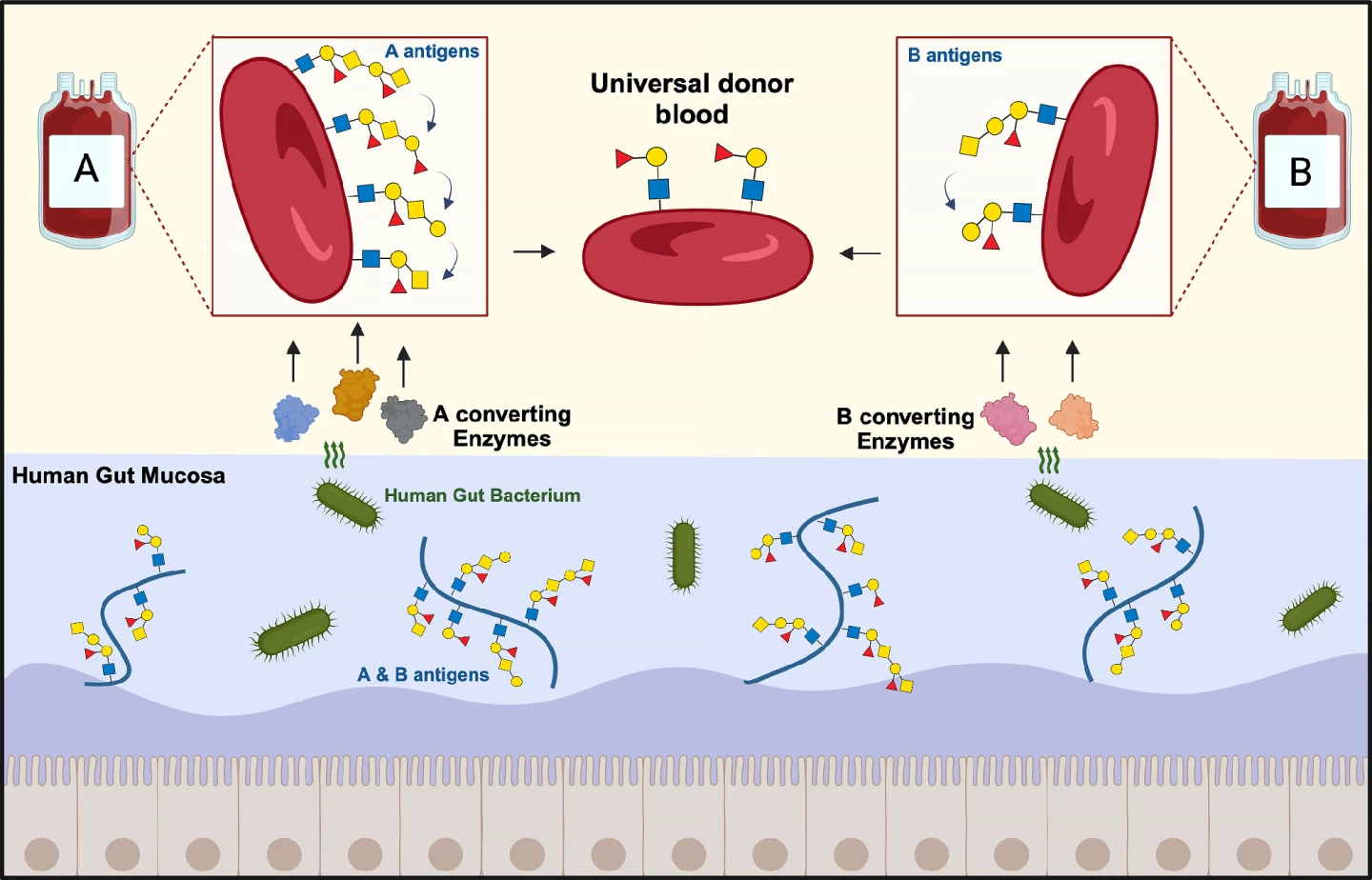
“What is special about the [intestinal] mucosa is that bacteria, which are able to live on this material, often have tailor-made enzymes to break down mucosal sugar structures, which include blood group ABO antigens,” Abou Hachem said.
The researchers tested 24 bacterial enzymes on hundreds of blood samples and found that they were highly efficient at converting A and B group blood into universal donor blood. Their action was more effective against B antigens than A antigens, with one candidate depleting B antigens to negative control levels.
“We are close to being able to produce universal blood from group B donors, while there is still work to be done to convert the more complex group A blood,” said Abou Hachem. “Our focus is now to investigate in detail if there are additional obstacles and how we can improve our enzymes to reach the ultimate goal of universal blood production.”
The researchers say that their findings have important implications for the future of blood transfusions.
“Universal blood will create a more efficient utilization of donor blood, and also avoid giving ABO-mismatched transfusions by mistake, which can otherwise lead to potentially fatal consequences in the recipient,” said Martin Olsson, the study’s other corresponding author. “When we can create ABO-universal donor blood, we will simplify the logistics of transporting and administering safe blood products, while at the same time minimizing blood waste.”
It's noted that the researchers do not mention the Rh factor in their study. In addition to the blood group, blood is further categorized as either Rh-positive (red blood cells carry the Rh antigen) or Rh-negative (they don’t). For example, someone might say they are 'AB negative'. The first transfusion of Rh-positive blood to a Rh-negative donor causes them to develop anti-Rh antibodies so that a subsequent transfusion may produce a reaction like that produced by giving group A blood to a group B person. Namely, the destruction of donor blood cells.
The researchers have applied for a patent on the new enzymes and their method of treatment. They expect to further their studies over the next three-and-a-half years before moving on to controlled patient trials.
The study was published in the journal Nature Microbiology.
Source: DTU