Researchers have used human tracheal cells to create tiny biological robots that can move on their own and work together to encourage healing in damaged neurons without requiring genetic modifications. The tiny bots have the potential to transform regenerative medicine and the treatment of disease.
Micro-sized robots created with living cells – biobots – are being developed to carry out various tasks inside the human body, from drug delivery to recognizing cancer cells. Now, researchers at Tufts University and Harvard’s Wyss Institute have taken a crack at creating their own biobot using human tracheal cells.
The researchers built upon previous work by Tufts, in collaboration with the University of Vermont, using frog embryo cells to create a multicellular biobot, called a Xenobot, capable of navigation, recording information and self-healing. At the time, researchers were unsure if these capabilities were because the Xenobot was made from frog cells or if a biobot could be constructed from cells from other species.
In the present study, the researchers wanted to see whether cells could be removed from their natural environment and recombined into different ‘body plans’ to carry out other functions. They discovered that bots could be created using adult human cells without genetic modification and with enhanced capabilities.
“We wanted to probe what cells can do besides create default features in the body,” said Gizem Gumuskaya, the study’s lead and corresponding author. “By reprogramming interactions between cells, new multicellular structures can be created, analogous to the way stone and brick can be arranged into different structural elements like walls, archways or columns.”
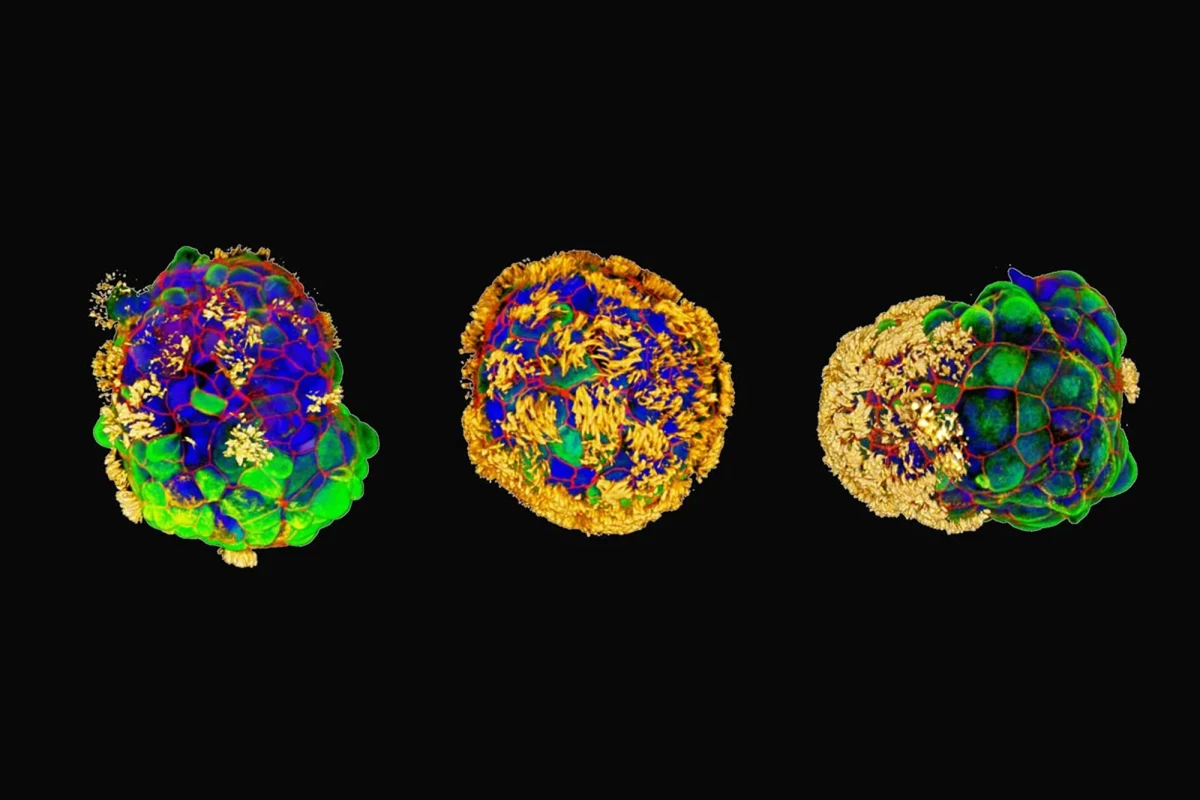
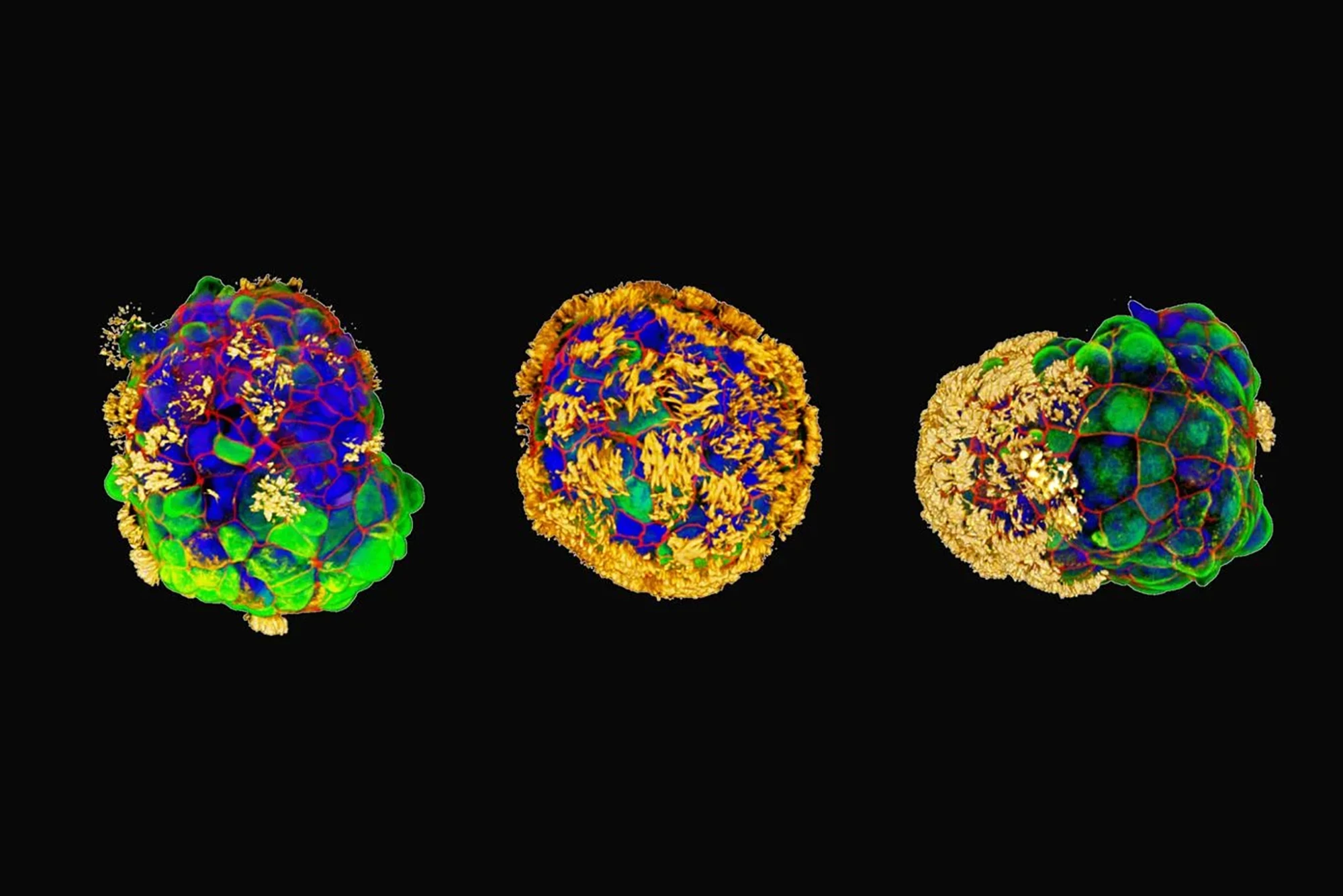
They began with tracheal cells taken from the surface of a human airway and developed a novel protocol that capitalizes on the existing ability of bronchial epithelial progenitor cells to form multicellular spheroids complete with cilia, microscopic hairlike structures that vibrate to move. They modified this process to yield cilia-coated spheroids; that is, the hairlike structures were on the outside rather than the inside.
Within a few days, the novel cells, which the researchers called Anthrobots, began moving, driven by the cilia. When fully grown, ranging in size from 30 to 500 microns, some bots were spherical and fully covered in cilia, while others were irregular or football-shaped with a patchy cilia covering. The distribution of cilia determined how the bots moved, either looping or wiggling in straight or curved paths. The Anthrobots usually survived for 45 to 60 days in laboratory conditions before they naturally biodegraded.
“Anthrobots self-assemble in the lab dish,” Gumuskaya said. “Unlike Xenobots, they don’t require tweezers or scalpels to give them shape, and we can use adult cells – even cells from elderly patients – instead of embryonic cells. It’s fully scalable – we can produce swarms of these bots in parallel, which is a good start for developing a therapeutic tool.”

The researchers grew a 2D layer of human neurons in a lab dish and scratched the cells with a thin metal rod to create a ‘wound’ devoid of cells. They placed a swarm of Anthrobots into the dish and observed them moving over the surface of the neurons. The bots encouraged new growth, filling the gap caused by the wound and creating a bridge of neurons as thick as the healthy cells. Neurons didn’t grow in the wound where the Anthrobots were absent.
“The cellular assemblies we construct in the lab can have capabilities that go beyond what they do in the body,” said Michael Levin, another corresponding author. “It is fascinating and completely unexpected that normal patient tracheal cells, without modifying their DNA, can move on their own and encourage neuron growth across a region of damage. We’re now looking at how the healing mechanism works and asking what else these constructs can do.”
An advantage of using human cells includes the ability to construct bots from a patient’s own cells to perform therapeutic work without triggering an immune response or requiring immunosuppressant medication.
Further development of the bots could lead to other applications, like clearing plaque buildup from arteries, repairing damaged spinal cords or retinal nerves, recognizing bacteria or cancer cells, or delivering drugs to targeted tissues. In theory, the Anthrobots could help heal tissue while delivering pro-regenerative drugs.
The study was published in the journal Advanced Science.
Sources: Tufts University, Wyss Institute